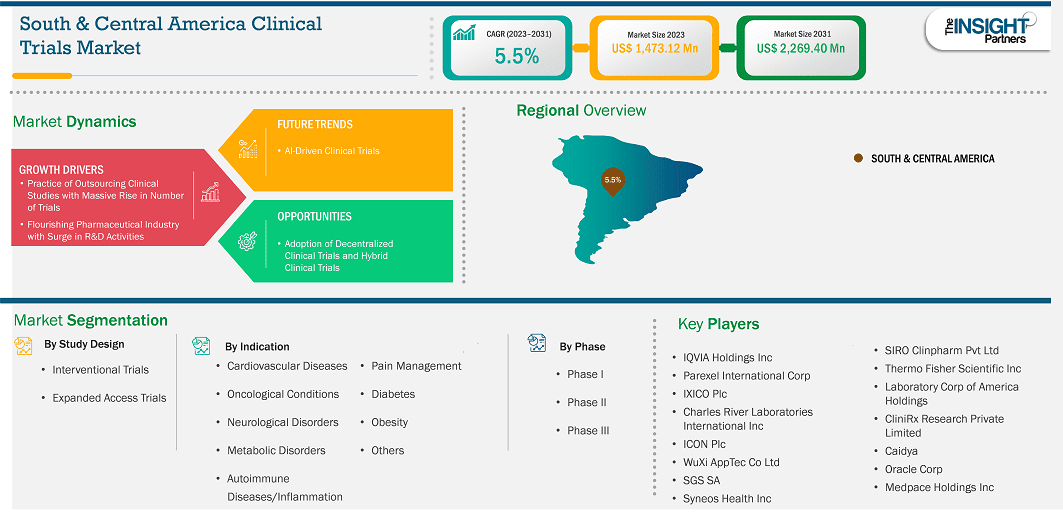
The South & Central America clinical trials market size is expected to reach US$ 2,269.40 million by 2031 from US$ 1,473.72 million in 2023. The market is estimated to record a CAGR of 5.5% from 2023 to 2031.
The South & Central America clinical trials market is growing steadily, driven by a diverse patient population and cost advantages. Increasing investments in healthcare infrastructure and regulatory improvements are attracting global sponsors. Despite progress, challenges such as limited access to advanced technology and varying regulatory standards across countries can slow market expansion.
South & Central America Clinical Trials Market Segmentation Analysis:
Key segments that contributed to the derivation of the South & Central America clinical trials market analysis are study design, indications, and phase type.
The adoption of artificial intelligence (AI) offers innovative ways to collect and manage clinical trial data, along with reducing dependency on manual operations. Thus, AI acts as a game changer for life science companies involved in the drug development process. AstraZeneca has collected oncology data, including clinical and imaging data, of more than 100,000 consenting patients for different clinical studies. The Oncology Data Science team at AstraZeneca feeds these data into a system that utilizes AI and other statistical tools to generate novel hypotheses for oncology drug development. To transform the process of oncology data feed, the team is adopting complex datasets for accessibility, interoperability, and reusability of data as per the GO FAIR principles, which emphasize on making data findable, accessible, interoperable, and reusable. Such integration empowers data collection from specific clinical trials and projects to be accessible across the company's drug development teams in compliance with data protection laws.
In November 2021, AstraZeneca collaborated with Tempus to leverage real-world data and represent patients globally. This strategic partnership focuses on offering crucial evidence about patient outcomes without revealing the identification of the clinical trial participants in the datasets. Moreover, with the power of AI, companies can rapidly digitize clinical trial processes to complete studies faster. Life-saving medicines and treatments can be provided to patients more quickly, and life sciences companies could gain a competitive edge. According to Deloitte's life sciences digital innovation survey 2023, 76% of respondents invested in AI for clinical development. Thus, AI-driven clinical trials, involving the use of AI for processing clinical trial data, are emerging as significant trends in the clinical trial market.
Based on country, the South & Central America clinical trials market comprises Brazil, Argentina, and the Rest of South & Central America. Brazil held the largest share in 2023.
Brazil is a significant destination for clinical trials. According to Clinical Trials Arena, Brazil accounted for a 1.7% share of the global clinical trials activity in 2021. Boston CRO acquired Rio de Janeiro-based Instituto Brasil de Pesquisa Clinica (IBPClin) in July 2022, describing it as the first step toward adopting its decentralized clinical trial delivery model in Latin America. It claims to have conducted over 160 industry-sponsored research studies, registering more than 7,000 participants across 12 Brazilian states. IBPClin is the largest research center on the continent. In March 2020, the Fiocruz Foundation built a new hospital center and invested in clinical trials with the WHO. The new hospital hosts the solidarity clinical trial led by Fiocruz in Brazil. The initiative is implemented in 18 hospitals in 12 states of Brazil with the support of the Department of Science and Technology of the Ministry of Health and coordinated by INI/Fiocruz. Thus, initiatives taken to develop clinical trials in Brazil boost the growth of the clinical trials market.
South & Central America Clinical Trials Market Company Profiles
Some of the key players operating in the market include QVIA Holdings Inc, Parexel International Corp, IXICO Plc, Charles River Laboratories International Inc, ICON Plc, WuXi AppTec Co Ltd, SGS SA, Syneos Health Inc, Thermo Fisher Scientific Inc, Laboratory Corp of America Holdings, CliniRx Research Private Limited, Caidya, Oracle Corp, Medpace Holdings Inc, and SIRO Clinpharm Pvt Ltd among others. These players are adopting various strategies such as expansion, product innovation, and mergers and acquisitions to provide innovative products to their consumers and increase their market share.
The following methodology has been followed for the collection and analysis of data presented in this report:
The research process begins with comprehensive secondary research, utilizing both internal and external sources to gather qualitative and quantitative data for each market. Commonly referenced secondary research sources include, but are not limited to:
Note: All financial data included in the Company Profiles section has been standardized to USD. For companies reporting in other currencies, figures have been converted to USD using the relevant exchange rates for the corresponding year.
The Insight Partners conducts a significant number of primary interviews each year with industry stakeholders and experts to validate its data analysis, and gain valuable insights. These research interviews are designed to:
Primary research is conducted via email interactions and telephone interviews, encompassing various markets, categories, segments, and sub-segments across different regions. Participants typically include:
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 1,473.72 Million |
| Market Size by 2031 | US$ 2,269.40 Million |
| CAGR (2023 - 2031) | 5.5% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Study Design
|
| Regions and Countries Covered |
South and Central America
|
| Market leaders and key company profiles |
|
The South & Central America Clinical Trials Market is valued at US$ 1,473.72 Million in 2023, it is projected to reach US$ 2,269.40 Million by 2031.
As per our report South & Central America Clinical Trials Market, the market size is valued at US$ 1,473.72 Million in 2023, projecting it to reach US$ 2,269.40 Million by 2031. This translates to a CAGR of approximately 5.5% during the forecast period.
The South & Central America Clinical Trials Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the South & Central America Clinical Trials Market report:
The South & Central America Clinical Trials Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The South & Central America Clinical Trials Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the South & Central America Clinical Trials Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)