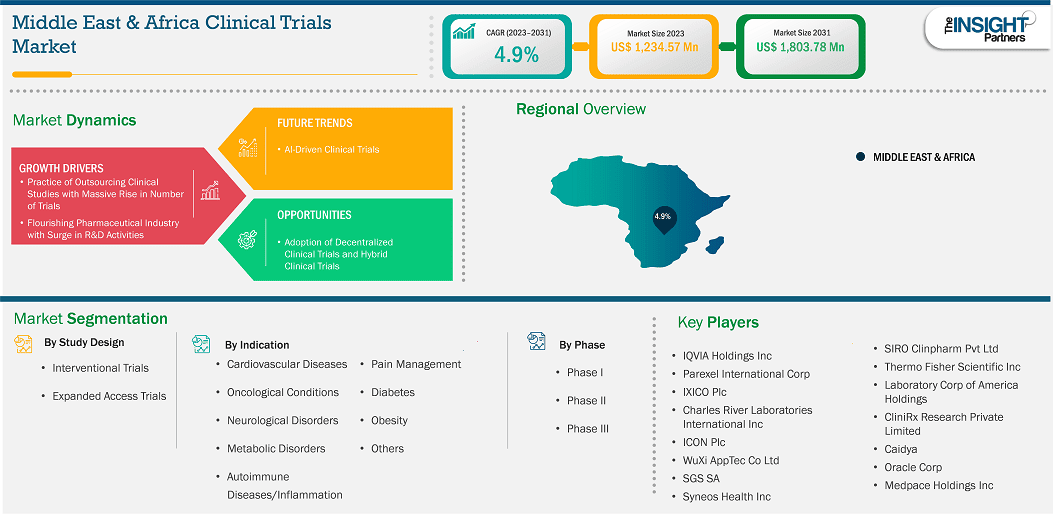
The Middle East & Africa clinical trials market size is expected to reach US$ 1,803.78 million by 2031 from US$ 1,234.57 million in 2023. The market is estimated to record a CAGR of 4.9% from 2023 to 2031.
Executive Summary and Middle East & Africa Clinical Trials Market Analysis:
The clinical trials market in the Middle East & Africa is segmented into Saudi Arabia, the UAE, South Africa, and the Rest of Middle East & Africa. The regional growth is attributed to rising rate of cancer and investments in developing healthcare infrastructure. Factors such as growing awareness of the advantages of clinical trials, government showing a propensity to spend on healthcare infrastructure and research are likely to further propel the clinical trials market growth during the forecast period.
Middle East & Africa Clinical Trials Market Segmentation Analysis:
Key segments that contributed to the derivation of the Middle East & Africa clinical trials market analysis are study design, indications, and phase type.
Clinical trials are conducted to determine if a new form of medical product, i.e., a drug, diet, or medical device, is safe and effective. The trials are mainly a part of the overall drug development process. According to the National Library of Medicine (NLM), ~52,000 new studies were registered with NLM (ClinicalTrials.gov) in 2020, and the number increased to ~58,000 in 2023. An upsurge in the number of clinical trials can be attributed to the rising prevalence of chronic diseases worldwide, which creates an immense demand for more effective treatments. The increasing complexity of clinical trials adds to the cruciality of the proper execution and overseeing of operations carried out in research-based organizations. To avoid errors due to improper execution, many research-based organizations outsource clinical trials to clinical research organizations (CROs). CROs assist in successfully implementing clinical trials through the services they offer through their high-quality facilities and deep subject matter expertise. They have gradually become a backbone of the clinical trial industry through their efficient and cost-effective operations that benefit trial sponsors. According to a blog published by Thermo Fisher Scientific, in 2022, CROs executed ~3 out of 4 clinical trials to reassure the clinical programs of drug developers, provide a wealth of expertise, drive time and cost efficiencies, and deliver customized, high-quality data. Thus, the increasing number of clinical trials, and the practice of outsourcing trials to CROs to boost cost-effectiveness and reduce errors are the major factors driving the clinical trials market growth.
Based on country, the Middle East & Africa clinical trials market comprises Saudi Arabia, South Africa, the UAE, and the Rest of Middle East & Africa. South Africa held the largest share in 2023.
Despite being one of the most economically advanced African nations, South Africa faces many public health challenges. TB is one of the leading causes of death in South Africa. According to the 2021 World Health Organization (WHO) data, South Africa ranked eighth among the 30 high-burden countries, contributing to 86% of the reported incident TB cases globally. UNAIDS estimates that ~7.8 million people in the country are currently suffering from HIV. To treat the increasing cases of HIV, various clinical trials are being conducted in the country.
According to an article titled "TB trials transform treatment," published in June 2022, research was conducted in South Africa to find shorter and more effective treatments for drug-resistant (DR) TB, which resulted in global policy changes. This research, conducted by the University of the Witwatersrand and the Clinical HIV Research Unit, formed part of clinical trials involving the Nix-TB and ZeNix studies conducted by the Global TB Alliance. Subsequently, WHO announced key changes to the treatment of DR-TB. Treatment time has been reduced from 18 months to 6 months, and the number of pills reduced from 23 a day to 23 per week, eliminating injections and reducing side effects.
Middle East & Africa Clinical Trials Market Company Profiles
Some of the key players operating in the market include QVIA Holdings Inc, Parexel International Corp, IXICO Plc, Charles River Laboratories International Inc, ICON Plc, WuXi AppTec Co Ltd, SGS SA, Syneos Health Inc, Thermo Fisher Scientific Inc, Laboratory Corp of America Holdings, CliniRx Research Private Limited, Caidya, Oracle Corp, Medpace Holdings Inc, and SIRO Clinpharm Pvt Ltd among others. These players are adopting various strategies such as expansion, product innovation, and mergers and acquisitions to provide innovative products to their consumers and increase their market share.
The following methodology has been followed for the collection and analysis of data presented in this report:
The research process begins with comprehensive secondary research, utilizing both internal and external sources to gather qualitative and quantitative data for each market. Commonly referenced secondary research sources include, but are not limited to:
Note: All financial data included in the Company Profiles section has been standardized to USD. For companies reporting in other currencies, figures have been converted to USD using the relevant exchange rates for the corresponding year.
The Insight Partners conducts a significant number of primary interviews each year with industry stakeholders and experts to validate its data analysis and gain valuable insights. These research interviews are designed to:
Primary research is conducted via email interactions and telephone interviews, encompassing various markets, categories, segments, and sub-segments across different regions. Participants typically include:
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 1,234.57 Million |
| Market Size by 2031 | US$ 1,803.78 Million |
| CAGR (2023 - 2031) | 4.9% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Study Design
|
| Regions and Countries Covered |
Middle East and Africa
|
| Market leaders and key company profiles |
|
The Middle East & Africa Clinical Trials Market is valued at US$ 1,234.57 Million in 2023, it is projected to reach US$ 1,803.78 Million by 2031.
As per our report Middle East & Africa Clinical Trials Market, the market size is valued at US$ 1,234.57 Million in 2023, projecting it to reach US$ 1,803.78 Million by 2031. This translates to a CAGR of approximately 4.9% during the forecast period.
The Middle East & Africa Clinical Trials Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Middle East & Africa Clinical Trials Market report:
The Middle East & Africa Clinical Trials Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Middle East & Africa Clinical Trials Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Middle East & Africa Clinical Trials Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)