The Nordic interventional cardiology devices market size is expected to reach US$ 665.6 million by 2033 from US$ 308.7 million in 2024. The market is estimated to record a CAGR of 8.9% from 2025 to 2033.
Executive Summary and Nordic Interventional Cardiology Devices Market Analysis:
The Nordic region, including Sweden, Denmark, Norway, and Finland, has a well-established interventional cardiology market, supported by strong public healthcare systems and early adoption of advanced medical technologies. High standards of care, widespread access to catheterization labs, and structured cardiac care programs enable consistent use of minimally invasive procedures such as stenting and valve interventions.
These countries show strong clinical uptake of intravascular imaging, pressure-guided diagnostics, and transcatheter heart valves. Robust reimbursement policies and government-supported innovation in cardiac care contribute to market stability. While the population size is relatively small, high procedure rates and consistent demand ensure that the Nordic region remains a high-value contributor within the broader European market.
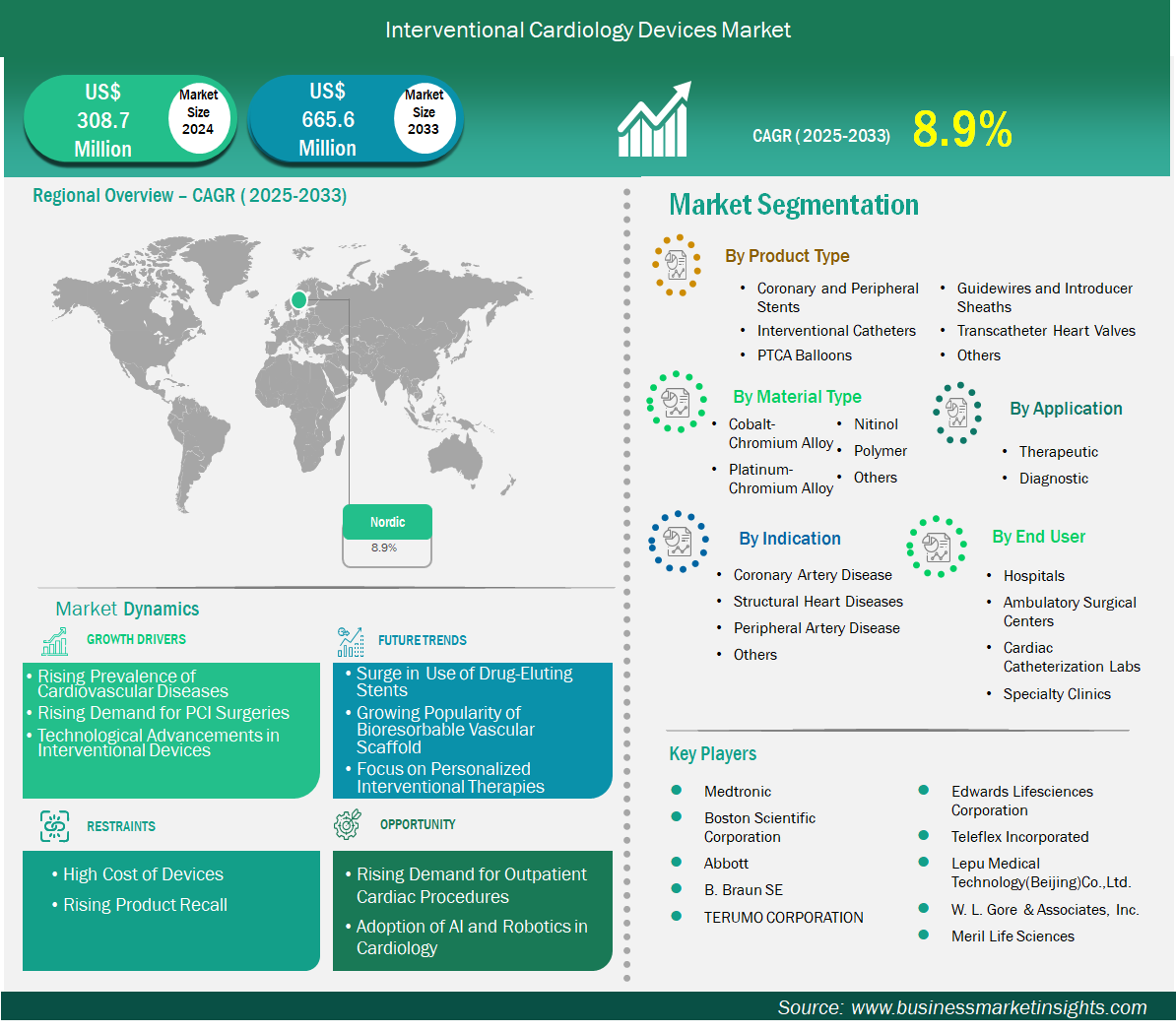
Key segments that contributed to the derivation of the interventional cardiology devices market analysis are product type, material type, application, indication, and end user.
Nordic Interventional Cardiology Devices Market Outlook
The interventional cardiology market in the Nordic countries is a highly mature and stable segment, defined by advanced healthcare systems and a focus on universal access. With a high standard of living and an aging population, the region faces a significant burden of cardiovascular diseases, ensuring a consistent and strong demand for cardiac interventions.
A key factor driving this market is the commitment to publicly funded healthcare and technological innovation. These countries have well-established, government-backed healthcare systems that prioritize quality, evidence-based care and ensure that every patient who needs an interventional procedure receives it. This consistent investment in healthcare, coupled with a propensity for rapid adoption of cutting-edge technologies such as next-generation drug-eluting stents and advanced diagnostic tools, creates a stable and valuable market for manufacturers.
Nordic Interventional Cardiology Devices Market Country Insights
Based on country, the Nordic interventional cardiology devices market is segmented into Sweden, Norway, Denmark, and Finland. Sweden held the largest share in 2024.
Sweden has a mature and innovation-driven interventional cardiology market, supported by a universal healthcare system, extensive hospital network, and a strong focus on clinical quality. Interventional procedures are widely available across major public hospitals and regional cardiac centers, with consistent use of advanced technologies such as drug-eluting stents, intravascular ultrasound, and radial access. Clinical decision-making is guided by national protocols aligned with European guidelines, emphasizing patient safety, procedural efficiency, and long-term outcomes. Sweden is highly active in international clinical trials and maintains comprehensive national registries, enabling evidence-based improvements in cardiac care. Procurement decisions prioritize cost-effectiveness and proven clinical value, though adoption of new technologies is generally timely in well-resourced centers. Digital health tools and remote diagnostics are gaining traction, especially in managing follow-ups and supporting care coordination. Although market size is moderate, Sweden’s emphasis on quality, innovation, and research makes it a leading Nordic market for interventional cardiology devices.
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 308.7 Million |
| Market Size by 2033 | US$ 665.6 Million |
| CAGR (2025 - 2033) | 8.9% |
| Historical Data | 2022-2023 |
| Forecast period | 2025-2033 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
Nordic
|
| Market leaders and key company profiles |
|
Medtronic, Boston Scientific Corporation, Abbott, B. Braun SE, TERUMO CORPORATION, Edwards Lifesciences Corporation, Teleflex Incorporated, Lepu Medical Technology (Beijing) Co., Ltd., W. L. Gore & Associates, Inc., and Meril Life Sciences are among the key players operating in the market. These players adopt strategies such as expansion, product innovation, and mergers and acquisitions to stay competitive in the market and offer innovative products to their consumers.
Nordic Interventional Cardiology Devices Market Research Methodology:
The following methodology has been followed for the collection and analysis of data presented in this report:
The research process begins with comprehensive secondary research, utilizing both internal and external sources to gather qualitative and quantitative data for each market. Commonly referenced secondary research sources include, but are not limited to:
Note: All financial data included in the Company Profiles section has been standardized to US$. For companies reporting in other currencies, figures have been converted to US$ using the relevant exchange rates for the corresponding year.
Business Market Insights conducts a significant number of primary interviews each year with industry stakeholders and experts to validate and analyze the data and gain valuable insights. These research interviews are designed to:
Primary research is conducted via email interactions and telephone interviews with industry experts across various markets, categories, segments, and sub-segments in different regions. Participants typically include:
The Nordic Interventional Cardiology Devices Market is valued at US$ 308.7 Million in 2024, it is projected to reach US$ 665.6 Million by 2033.
As per our report Nordic Interventional Cardiology Devices Market, the market size is valued at US$ 308.7 Million in 2024, projecting it to reach US$ 665.6 Million by 2033. This translates to a CAGR of approximately 8.9% during the forecast period.
The Nordic Interventional Cardiology Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Nordic Interventional Cardiology Devices Market report:
The Nordic Interventional Cardiology Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Nordic Interventional Cardiology Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Nordic Interventional Cardiology Devices Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)