The Europe interventional cardiology devices market size is expected to reach US$ 9,849.28 million by 2033 from US$ 5,076.43 million in 2024. The market is estimated to record a CAGR of 7.8% from 2025 to 2033.
Executive Summary and Europe Interventional Cardiology Devices Market Analysis:
Europe holds significant share in the global insterventional cardiology devices market, supported by advanced healthcare systems, widespread access to cardiac care, and a high prevalence of cardiovascular diseases. Major countries such as Germany, France, the United Kingdom, Italy, and Spain lead regional demand, with well-established hospital networks and a strong focus on minimally invasive cardiac procedures.
The region has seen significant adoption of technologies such as drug-eluting stents, transcatheter heart valves, and intravascular imaging tools. Europe’s well-regulated healthcare environment encourages the early adoption of innovative devices, supported by clinical guidelines and reimbursement systems. Increasing geriatric population and lifestyle-related risk factors continue to drive the need for coronary and peripheral interventions.
There is also growing interest in day-care procedures and the expansion of cardiac catheterization labs across the region. In addition, ongoing research collaborations, government funding for cardiovascular care, and focus on reducing surgical burden are fueling growth in interventional therapies.
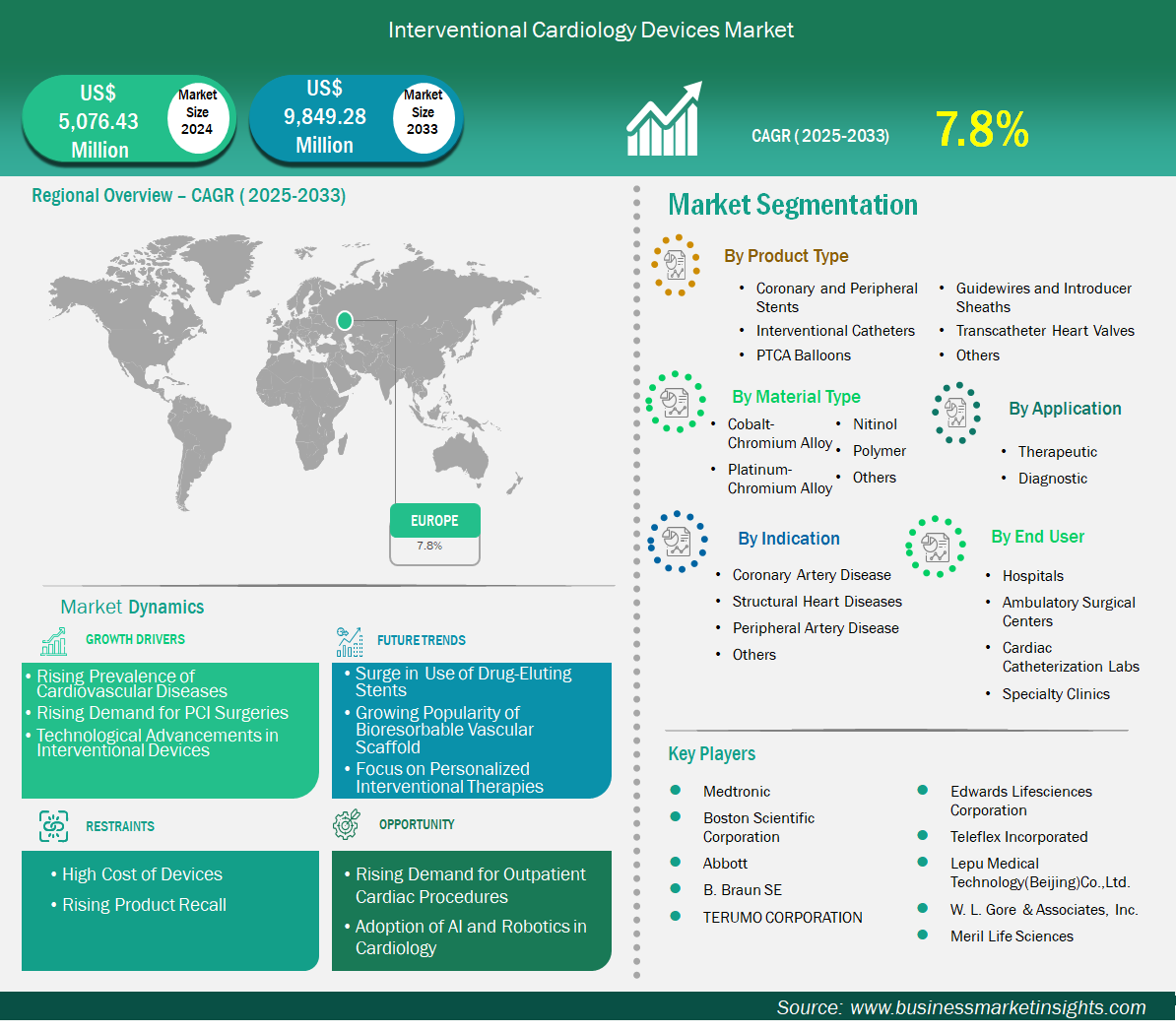
Key segments that contributed to the derivation of the interventional cardiology devices market analysis are product type, material type, application, indication, and end user.
Europe Interventional Cardiology Devices Market Outlook
The European interventional cardiology market is a highly mature and established segment, driven by a combination of advanced healthcare infrastructure and an aging population. Europe has one of the world's oldest populations, with a high and growing prevalence of chronic conditions, particularly cardiovascular diseases. This demographic reality creates a substantial and consistent demand for cardiac interventions.
A key factor propelling the market is the region's high standards of healthcare and technological adoption. European countries, especially in the west, have well-funded public and private healthcare systems that readily invest in and adopt the latest medical technologies. The quick integration of next-generation devices, such as advanced drug-eluting stents and sophisticated imaging catheters, is a hallmark of the European market, ensuring that patients receive cutting-edge care and sustaining market growth.
Europe Interventional Cardiology Devices Market Country Insights
Based on country, the Europe interventional cardiology devices market is segmented into the Germany, Italy, France, the U.K., Spain, Belgium, the Netherlands, Luxembourg, Norway, Finland, Denmark, Sweden, Austria, Switzerland, Russia, Romania, Greece, Czech Republic, Portugal, Ukraine, Poland, Slovakia, and Bulgaria. Germany held the largest share in 2024.
Germany dominates the European market in the interventional cardiology device landscape, characterized by a sophisticated healthcare system, a high volume of cardiac procedures, and a strong adoption rate of advanced medical technologies. The country's robust economy and comprehensive health insurance coverage further contribute to its leading position.
Cardiovascular diseases remain the leading cause of death in Germany, accounting for approximately 40% of all fatalities. This significant disease burden drives a continuous demand for diagnostic and interventional solutions. In 2022, the total number of interventional cardiology procedures performed in Germany was over 1.47 million. Germany also ranked high in Europe for transluminal coronary angioplasty procedures, with 379.3 procedures per 100,000 inhabitants in 2022, indicating a strong reliance on PCI for CAD treatment.
Furthermore, Germany is a key market for the adoption of innovative products. The presence of major medical device companies and a strong research environment ensure that new technologies are rapidly integrated into clinical practice. This blend of high disease prevalence, advanced medical infrastructure, and a proactive approach to adopting cutting-edge interventional techniques solidifies Germany's role as a driving force in the global interventional cardiology devices market.
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 5,076.43 Million |
| Market Size by 2033 | US$ 9,849.28 Million |
| CAGR (2025 - 2033) | 7.8% |
| Historical Data | 2022-2023 |
| Forecast period | 2025-2033 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
Europe
|
| Market leaders and key company profiles |
|
Medtronic, Boston Scientific Corporation, Abbott, B. Braun SE, TERUMO CORPORATION, Edwards Lifesciences Corporation, Teleflex Incorporated, Lepu Medical Technology (Beijing) Co., Ltd., W. L. Gore & Associates, Inc., and Meril Life Sciences are among the key players operating in the market. These players adopt strategies such as expansion, product innovation, and mergers and acquisitions to stay competitive in the market and offer innovative products to their consumers.
Europe Interventional Cardiology Devices Market Research Methodology:
The following methodology has been followed for the collection and analysis of data presented in this report:
The research process begins with comprehensive secondary research, utilizing both internal and external sources to gather qualitative and quantitative data for each market. Commonly referenced secondary research sources include, but are not limited to:
Note: All financial data included in the Company Profiles section has been standardized to US$. For companies reporting in other currencies, figures have been converted to US$ using the relevant exchange rates for the corresponding year.
Business Market Insights conducts a significant number of primary interviews each year with industry stakeholders and experts to validate and analyze the data and gain valuable insights. These research interviews are designed to:
Primary research is conducted via email interactions and telephone interviews with industry experts across various markets, categories, segments, and sub-segments in different regions. Participants typically include:
The Europe Interventional Cardiology Devices Market is valued at US$ 5,076.43 Million in 2024, it is projected to reach US$ 9,849.28 Million by 2033.
As per our report Europe Interventional Cardiology Devices Market, the market size is valued at US$ 5,076.43 Million in 2024, projecting it to reach US$ 9,849.28 Million by 2033. This translates to a CAGR of approximately 7.8% during the forecast period.
The Europe Interventional Cardiology Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Interventional Cardiology Devices Market report:
The Europe Interventional Cardiology Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Interventional Cardiology Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Interventional Cardiology Devices Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)