With the increasing number of medical devices used in patient care, the ability to collect, manage, and use data from these devices has become more complex. Medical device connectivity refers to linking medical devices to electronic health records (EHRs) or electronic medical records (EMRs) to facilitate data exchange. An EHR is a digital version of a patient’s paper chart. EHRs are real-time, patient-centered records that make information available instantly and securely to authorized users. An EHR system is built to include standard clinical data collected in a provider’s office as well as detailed information on a patient’s care. Integrating medical device and equipment data with EMR systems and clinical solutions is critical to modern healthcare. Initially, when the concept of EMR was introduced, the hospital staff had to manually store and check the data from the medical device, which was time-consuming. With the use of medical device connectivity, the data is transferred directly from a medical device to the EHR/EMR software, reducing the time from ~4 minutes to 20 seconds. Also, the integration is crucial for providing a comprehensive and up-to-date view of a patient’s health information, enabling providers to quickly make informed decisions and deliver better patient care, as well as lowering healthcare costs. With the rise of wearable and portable medical devices and new medical equipment, healthcare providers increasingly integrate vast amounts of data into their EMR systems. The high adoption of EMR solutions and the growing emphasis of governments across various developed and developing countries on building nationwide healthcare information exchanges are expected to increase the demand for efficient medical device connectivity solutions. Thus, the growing use of EHR/EMR is fueling the growth of the Europe medical device connectivity market.
The Europe medical device connectivity market is segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. The region holds a significant market share in the medical device connectivity market. There is increasing interest among EU institutions, national governments, healthcare industries, and stakeholders to digitalize the healthcare ecosystem. This aims at deploying more cost-effective healthcare provisions, reducing the frontline nursing workload, easing lifelong learning activities for healthcare professionals, facilitating cross-border care, and fully developing the EU’s Electronic Healthcare Records (EHR). In 2022, a new report by the European Commission, led by the renowned UOC’s spin-off company Open Evidence, examined the implementation of the electronic health record in the European Union countries, Norway, and the UK, as well as the current degree of interoperability. According to the report, while most countries have established digital health record systems, an interoperable EHR is incorporated in most of the systems that were studied, and many patients are unable to access and use or transfer their data between healthcare providers. The healthcare systems in Europe are organized in varied ways; for instance, they are centralized, decentralized, dependent on public insurers, private, etc. Hence, it is challenging to create a definitive profile, as there are interoperable systems between some regions and countries, such as Estonia and Finland.
Strategic insights for the Europe Medical Device Connectivity provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Medical Device Connectivity refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Europe Medical Device Connectivity Strategic Insights
Europe Medical Device Connectivity Report Scope
Report Attribute
Details
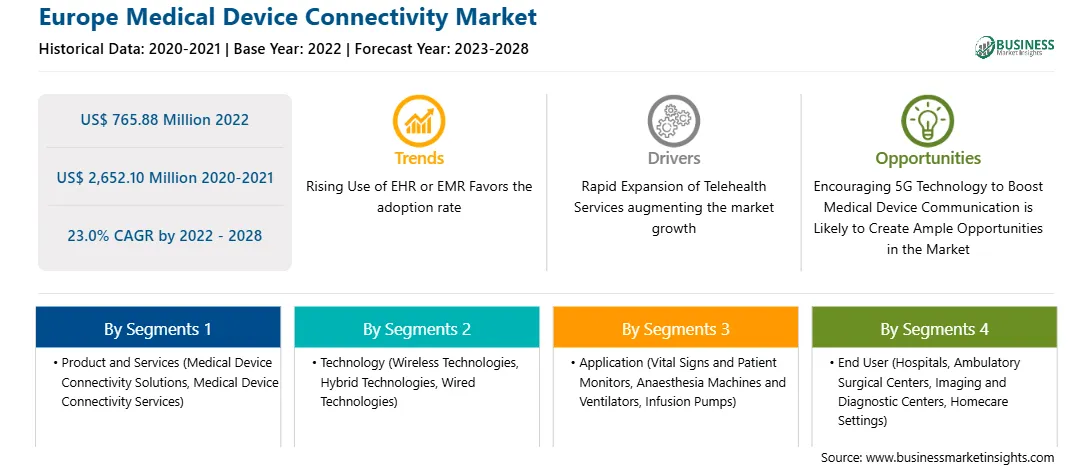
Market size in 2022
US$ 765.88 Million
Market Size by 2028
US$ 2,652.10 Million
CAGR (2022 - 2028) 23.0%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Product and Services
By Technology
By Application
By End User
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Medical Device Connectivity Regional Insights
Europe Medical Device Connectivity Market Segmentation
The Europe medical device connectivity market is segmented on the basis of product and services, technology, application, end user, and country. Based on product and services, the Europe medical device connectivity market is bifurcated into medical device connectivity solutions and medical device connectivity services. The medical device connectivity solutions segment registered a larger market share in 2022.
Based on technology, the Europe medical device connectivity market is segmented into wireless technologies, hybrid technologies, and wired technologies. The wireless technologies segment registered the largest market share in 2022.
Based on application, the Europe medical device connectivity market is segmented into vital signs and patient monitors, anesthesia machines and ventilators, infusion pumps, and others. The vital signs and patient monitors segment registered the largest market share in 2022.
Based on end user, the Europe medical device connectivity market is segmented into hospitals, ambulatory surgical centers, imaging and diagnostic centers, and homecare settings. The hospitals segment registered the largest market share in 2022.
Based on country, the Europe medical device connectivity market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. Germany dominated the market share in 2022.
Cisco Systems Inc, Digi International Inc., GE HealthCare Technologies Inc, iHealth Labs Inc, Infosys Ltd, Koninklijke Philips NV, Lantronix Inc., Medtronic Plc, Oracle Corp, and Silicon & Software Systems Ltd are the leading companies operating in the Europe medical device connectivity market.
The Europe Medical Device Connectivity Market is valued at US$ 765.88 Million in 2022, it is projected to reach US$ 2,652.10 Million by 2028.
As per our report Europe Medical Device Connectivity Market, the market size is valued at US$ 765.88 Million in 2022, projecting it to reach US$ 2,652.10 Million by 2028. This translates to a CAGR of approximately 23.0% during the forecast period.
The Europe Medical Device Connectivity Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Medical Device Connectivity Market report:
The Europe Medical Device Connectivity Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Medical Device Connectivity Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Medical Device Connectivity Market value chain can benefit from the information contained in a comprehensive market report.