The Europe embolization devices market size is expected to reach US$ 1,671.99 million by 2031 from US$ 886.97 million in 2024. The market is estimated to record a CAGR of 9.6% from 2025 to 2031.
The Europe embolization devices market is further segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. The region occupies a significant position in the overall market and is expected to record a strong growth rate during the forecast period. The market growth in the region is mainly attributed to the burgeoning cases of colorectal cancer and gastrointestinal bleeding.
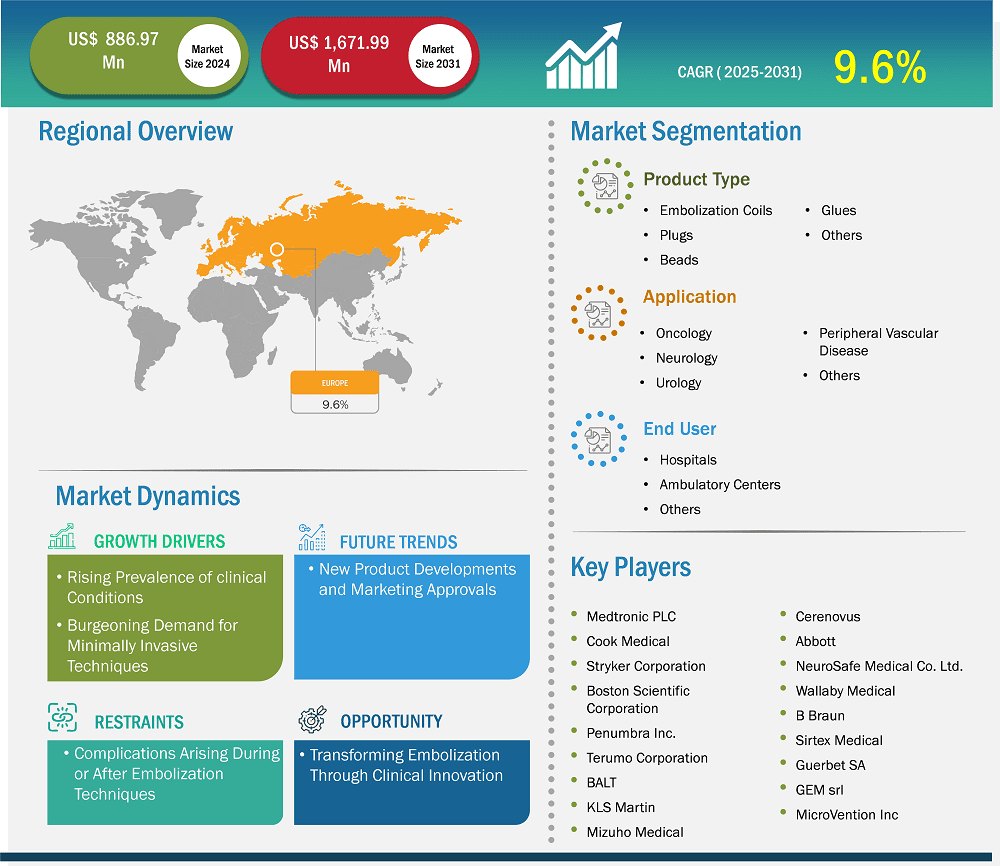
Key segments that contributed to the derivation of the Europe embolization devices market analysis are type, application, and end user.
Small and big companies operating in the embolization devices market are increasingly adopting strategies such as geographic expansions, new product developments, and technological advancements to boost their revenues. A few of the noteworthy product developments and approvals in the embolization devices market, which are expected to drive its growth in the coming years, are mentioned below.
Therefore, new product developments and marketing approvals are anticipated to create lucrative growth opportunities in the embolization devices market.
Based on country, the Europe embolization devices market comprises Germany, the UK, France, Italy, Spain, and the Rest of Europe. Germany held the largest share in 2024.
The embolization devices market growth in Germany is driven by a strong healthcare system, significant investments in medical research, and the early adoption of minimally invasive technologies. According to GLOBOCAN 2022, Germany recorded ~62,544 new colorectal cases (including 29,347 women and 33,197 men) in 2022; nearly 1 in 8 of these cases show cancerous growth in the large intestine (colon) or rectum in clinical evaluation. Hospitals and clinics in the country are well-equipped with interventional radiology departments that frequently utilize embolization techniques for treating conditions such as aneurysms, arteriovenous malformations, and tumors. The healthcare sector in the country also benefits from a well-organized reimbursement framework that supports the use of innovative procedures, encouraging the adoption of embolization therapies on a broader scale.
Germany is home to leading academic institutions and research centers that partner with global MedTech companies to develop and refine embolization technologies. For instance, a research team from Charité – University Hospital Berlin was awarded the RSNA 2024 Trainee Research Prize for their retrospective, single-center study involving 403 patients aged 40–90 (median age 69) with moderate to severe knee osteoarthritis who underwent genicular artery embolization (GAE). The study assessed the safety and effectiveness of this novel interventional procedure for managing symptoms associated with knee osteoarthritis. Radiology departments in Germany also have well-established training and certification programs for interventional radiologists in place, ensuring high procedural quality and patient outcomes. There is also a growing trend toward using embolization in oncology, particularly for treating liver and uterine cancers, due to its targeted approach and lower risk profile compared to surgery.
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 886.97 Million |
| Market Size by 2031 | US$ 1,671.99 Million |
| CAGR (2025 - 2031) | 9.6% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
Europe
|
| Market leaders and key company profiles |
|
Some of the key players operating in the market include Medtronic Plc; Cook Medical Holdings LLC; Stryker Corp; Boston Scientific Corp; Terumo Corp; Johnson & Johnson; Abbott Laboratories; NeuroSafe Medical Co. Ltd; Wallaby Medical; Sirtex Medical Ltd; GEM srl; Penumbra Inc.; Balt; Lepu Medical Technology Beijing Co Ltd; INVAMED; Meril Life Sciences Pvt Ltd; Merit Medical Systems Inc; and Lifetech Scientific Corp, among others. These players are adopting various strategies such as expansion, product innovation, and mergers and acquisitions to provide innovative products to their consumers and increase their market share.
The following methodology has been followed for the collection and analysis of data presented in this report:
The research process begins with comprehensive secondary research, utilizing both internal and external sources to gather qualitative and quantitative data for each market. Commonly referenced secondary research sources include, but are not limited to:
Note: All financial data included in the Company Profiles section has been standardized to USD. For companies reporting in other currencies, figures have been converted to USD using the relevant exchange rates for the corresponding year.
The Insights Partners conducts a significant number of primary interviews each year with industry stakeholders and experts to validate its data analysis and gain valuable insights. These research interviews are designed to:
Primary research is conducted via email interactions and telephone interviews, encompassing various markets, categories, segments, and sub-segments across different regions. Participants typically include:
The Europe Embolization Devices Market is valued at US$ 886.97 Million in 2024, it is projected to reach US$ 1,671.99 Million by 2031.
As per our report Europe Embolization Devices Market, the market size is valued at US$ 886.97 Million in 2024, projecting it to reach US$ 1,671.99 Million by 2031. This translates to a CAGR of approximately 9.6% during the forecast period.
The Europe Embolization Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Embolization Devices Market report:
The Europe Embolization Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Embolization Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Embolization Devices Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)