The poll conducted by Pew Research Center stated that 52% of the US adults do not favor animal use in scientific research. Additionally, other surveys found rest of the population accept animal experimentation is because they believe it to be necessary for medical research growth. Thus, the growing concerns related to the harm caused to animals during different clinical trials have created a demand for alternative methods for carrying out these trials, such as in-silico trials. In-silico clinical trials have overcome the disadvantages of animal testing and are considered a better method due to high accuracy, low cost, and time efficiency. In-silico clinical trials offer an effective way of replacing animal anatomies for conducting various experiments and research & development activities, thus, supporting the growth of the in-silico trials market during the forecast period. Additionally, rapidly increasing concerns over animal welfare and consistent efforts of human and animal rights and welfare organizations are boosting the growth of the North America in-silico trials market.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market at a promising CAGR during the forecast period.
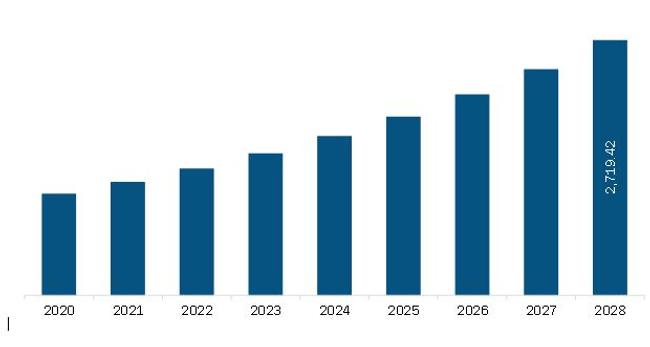
The North America in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented on the basis of organization size, offerings, application, clinical indication, and end user. Based on organization size, the market is bifurcated into small and medium organizations and large organizations. Based on offerings, the North America in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into products, platforms, and services. Based on application, the market is segmented into product design and discovery, product development, pre-clinical targeting, assessment of drugs and other biomedical products, and others. Based on clinical indication, the market is segmented into cardiovascular diseases, neurodegenerative diseases, oncology, rare diseases, metabolic diseases, immune based diseases, infectious diseases, and others. Based on end user, the market is segmented into pharmaceutical and biopharmaceutical companies, medical technology companies, contract research organizations, and others. By country, the North America in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into the US, Canada, and Mexico.
InSilicoTrials Technologies; Dassault Systèmes SE; Certara Inc.; Computational Life; NOVA; Ansys, Inc.; Synopsys, Inc.; Sensyne Health plc.; Phesi; Tempus; and Cerner Corporation are among the leading companies in the North America in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market.
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 1,204.4 Million |
| Market Size by 2028 | US$ 2,719.4 Million |
| CAGR (2021 - 2028) | 12.3% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Organization Size
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|

The North America In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market is valued at US$ 1,204.4 Million in 2021, it is projected to reach US$ 2,719.4 Million by 2028.
As per our report North America In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, the market size is valued at US$ 1,204.4 Million in 2021, projecting it to reach US$ 2,719.4 Million by 2028. This translates to a CAGR of approximately 12.3% during the forecast period.
The North America In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market report:
The North America In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)