In-silico clinical trials can supplement phase II drug trials to explore the safety and efficacy in the more infrequent phenotypes that usually appear only in phase III and predict the dose-effect relationship. Computational study in natural drug discovery is used to determine the metabolic pathways of active molecules and find the new targets and new molecules with high affinity to those targets. For instance, camptothecin derivatives (monoterpene-indole alkaloids) have been clinically employed as antitumor drugs. Such studies may explain how an in-silico metabolic analysis can improve the experimental decorations to gain more comprehensible biological information. Another aspect of human trials is that they are expensive, lengthy, and are typically designed with a narrow scope. But in-silico trials can be designed to answer a wide variety of relevant questions by providing sufficient statistical power to test the different hypotheses with a relatively small increase in resources associated with evaluating more models once the computational machinery is developed and implemented.
Furthermore, unlike conventional clinical trials, in-silico clinical trials are readily approved and authorized by the food and drug administration (FDA). Incrementally but inevitably, the in-silico clinical trial approach is expected to become the most significant evidence for regulatory evaluations. These factors indicate that the benefits mentioned above are substantially driving the in-silico trial market in Europe.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the Europe in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market at a promising CAGR during the forecast period.
The market for in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into organization size, offerings, application, clinical indication, and end user. Based on organization size, the Europe in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into small and medium organizations and large organizations. Based on offerings, the Europe in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into products, platforms, and services. Based on application, the Europe in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into product design and discovery, product development, pre-clinical targeting, assessment of drugs and other biomedical products, and others. Based on clinical indication, the Europe in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into cardiovascular diseases, neurodegenerative diseases, oncology, rare diseases, metabolic diseases, immune based diseases, infectious diseases, and others. Based on end user, the in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into pharmaceutical and biopharmaceutical companies, medical technology companies, contract research organizations, and others. By country, the Europe in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into Germany, the UK, France, Spain, Italy, and the rest of Europe.
InSilicoTrials Technologies; Feops; CADFEM Medical GmbH; Dassault Systèmes SE; Virtonomy GmbH; Certara Inc.; Computational Life; NOVA; TwInsight Medical; Ansys, Inc.; Synopsys, Inc.; Sensyne Health plc.; and Cerner Corporation are among the leading companies in the Europe in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market.
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 816.0 Million |
| Market Size by 2028 | US$ 1,870.3 Million |
| CAGR (2021 - 2028) | 12.6% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Organization Size
|
| Regions and Countries Covered |
Europe
|
| Market leaders and key company profiles |
|

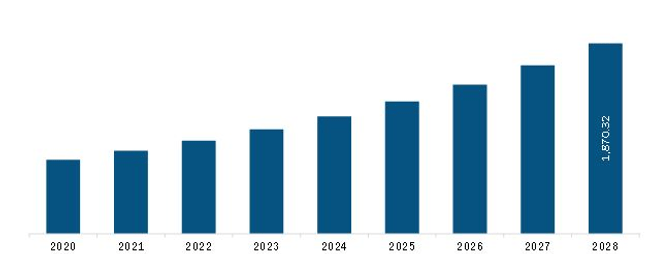
The Europe In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market is valued at US$ 816.0 Million in 2021, it is projected to reach US$ 1,870.3 Million by 2028.
As per our report Europe In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, the market size is valued at US$ 816.0 Million in 2021, projecting it to reach US$ 1,870.3 Million by 2028. This translates to a CAGR of approximately 12.6% during the forecast period.
The Europe In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market report:
The Europe In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)