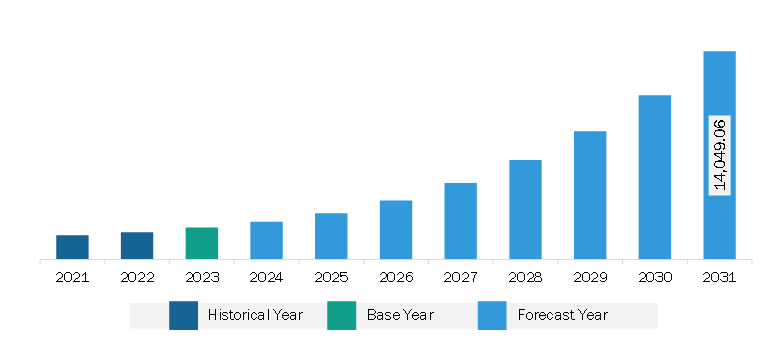
The North America cell and gene therapy market was valued at US$ 2,159.08 million in 2023 and is projected to reach US$ 14,049.06 million by 2031; it is estimated to record a CAGR of 26.4% from 2023 to 2031.
Increase in Number of Approval of Cell and Gene Therapies Boosts North America Cell and Gene Therapy Market
The advancements in biotechnology have led to the adoption of personalized treatments for a wide range of indications. Stem cell therapies are being used to treat cancer, neurological disorders, genetic disorders, and other chronic diseases. Further, the advantages of cell therapy include targeted treatment, rapid and efficient recovery, and reduced side effects. Cell therapies are widely adopted worldwide owing to the availability of Food and Drug Administration (FDA) approved products. A few of the cell and gene therapy products approved by the FDA in recent years are mentioned; In April 2024, the FDA approved BEQVEZ for use by Pfizer Inc. to treat adults suffering from moderate to severe hemophilia B who are on factor IX (FIX) prophylaxis therapy. A FIX deficiency causes people with hemophilia B, a rare genetic bleeding illness, to bleed more frequently and for longer periods than healthy people. The disease hinders normal blood clotting. In 2023, the FDA approved VYJUVEK, manufactured by Krystal Biotech, Inc., for the treatment of wounds in patients ages 6 months and above with dystrophic epidermolysis bullosa, showing mutation(s) in the collagen type VII alpha 1 chain (COL7A1) gene. In 2023, ADSTILADRIN, an adenovirus manufactured by Ferring Pharmaceuticals A/S, was approved by the FDA. This recombinant adenovirus (rAd-IFNa/Syn3) delivers human interferon alfa-2b cDNA into the bladder epithelium to treat patients with certain types of bladder cancer. In 2023, CARVYKTI, manufactured by Janssen Biotech, Inc.-an autologous CAR-T cell engineered with lentivirus to attack BCMA-expressing tumor cells for the treatment of certain kinds of relapsed or refractory multiple myeloma-was also approved by the FDA. In 2023, the FDA approved HEMGENIX, manufactured by CSL Behring LLC, which is a recombinant AAV5 that delivers F9 to treat patients with certain kinds of Hemophilia B. In March 2021, the first anti-BCMA CAR T cell therapy for relapsed or resistant multiple myeloma, called Abecma (idecabtagene vicleucel), has been approved by the US FDA for use by Bristol Myers Squibb and Bluebird Bio. In April 2020, the FDA awarded regenerative medicine advanced therapy designation to Novartis' Kymriah to treat refractory (r/r) follicular lymphoma (FL) in adults. In July 2020, the FDA approved a CAR T-cell therapy brexucabtagene autoleucel (Tecartus) for patients with mantle cell lymphoma. It is the first FDA-approved CAR T-cell therapy for mantle cell lymphoma, and it was approved under the accelerated approval pathway. Tecartus also received Orphan Drug designation, which encourages the development of drugs for rare diseases. The other approved CAR-T cell therapies for cancer are Kymriah for acute lymphoblastic leukemia and Yescarta for diffuse large B-cell lymphoma. Therefore, the increasing number of approvals of cell and gene therapies enhances manufacturing capabilities, which fuels the North America cell and gene therapy market growth.
North America Cell and Gene Therapy Market Overview
The cell and gene therapy market in North America is segmented into the US, Canada, and Mexico. The US held the largest North American cell and gene therapy market share in 2023. The cell and gene therapies market growth in the US is attributed the growing adoption of cell therapies such as stem cell, gene, and immune therapies. Growing incidences of genetic and cellular disorders are leading to increasing demand for cell therapies. According to the American Society of Gene & Cell Therapy (ASGCT), there are currently 3,633 therapies in the pipeline-55% are gene, 22% are non-genetically modified cells, and 23% are RNA-from preclinical through pre-registration. These are focused on various diseases and conditions varying from cancer to genetic disorders to neurological conditions. As of February 2024, 19 cell and gene therapy products have been approved in the US for treating cancer, eye diseases, and rare hereditary diseases. Also, the country is experiencing an increasing number of start-ups innovating cell therapies. In addition, growing support from the government is promoting the growth of cell therapies, influencing the development of the market. For instance, the American Society of Gene & Cell Therapy (ASGCT), a public organization, offers memberships to scientists, physicians, professionals, and patient advocates who are engaged in gene and cell therapies. ASGCT aims to enhance knowledge, education, and awareness regarding the clinical application of cell and gene therapies.
North America Cell and Gene Therapy Market Revenue and Forecast to 2031 (US$ Million)
North America Cell and Gene Therapy Market Segmentation
The North America cell and gene therapy market is categorized into type, services, scale, end user, and country.
Based on type, the North America cell and gene therapy market is bifurcated into cell therapy and gene therapy. The cell therapy segment held a larger market share in 2023. Furthermore, the cell therapy segment is sub segmented into allogeneic, autologous, viral vectors. Additionally, the gene therapy segment is divided into non-viral vectors and viral vectors.
In terms of services, the North America cell and gene therapy market is categorized process development, cGMP manufacturing, regulatory services, and bioassay services. The process development segment held the largest market share in 2023.
By scale, the North America cell and gene therapy market is bifurcated into pre-commercial/ R&D manufacturing and commercial scale manufacturing. The pre-commercial/ R&D manufacturing segment held a larger market share in 2023.
Based on end user, the North America cell and gene therapy market is segmented into contract research organizations, pharmaceutical and biopharmaceutical companies, and academic and research institutes. The contract research organizations segment held the largest market share in 2023.
By country, the North America cell and gene therapy market is segmented into the US, Canada, and Mexico. The US dominated the North America cell and gene therapy market share in 2023.
Catalent Inc., Charles River Laboratories International Inc., F. Hoffmann-La Roche Ltd, FUJIFILM Holdings Corp, Lonza Group AG, Lotte Corp, Merck KGaA, Takara Bio Inc, Thermo Fisher Scientific Inc., and WuXi AppTec Co Ltd are some of the leading companies operating in the North America cell and gene therapy market.

| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 2,159.08 Million |
| Market Size by 2031 | US$ 14,049.06 Million |
| CAGR (2023 - 2031) | 26.4% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|

The North America Cell and Gene Therapy Market is valued at US$ 2,159.08 Million in 2023, it is projected to reach US$ 14,049.06 Million by 2031.
As per our report North America Cell and Gene Therapy Market, the market size is valued at US$ 2,159.08 Million in 2023, projecting it to reach US$ 14,049.06 Million by 2031. This translates to a CAGR of approximately 26.4% during the forecast period.
The North America Cell and Gene Therapy Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Cell and Gene Therapy Market report:
The North America Cell and Gene Therapy Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Cell and Gene Therapy Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Cell and Gene Therapy Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)