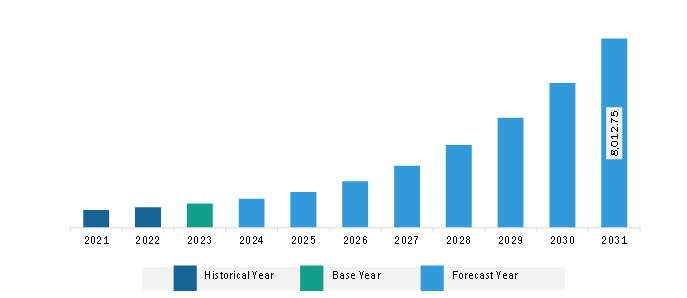
The Asia Pacific cell and gene therapy market was valued at US$ 1,024.37 million in 2023 and is projected to reach US$ 8,012.75 million by 2031; it is estimated to record a CAGR of 29.3% from 2023 to 2031.
Strategic Initiatives by Companies Drive Asia Pacific Cell and Gene Therapy Market
Many companies involved in manufacturing cell and gene therapy products focus on collaborations, expansions, agreements, partnerships, new product launches, and other strategic developments. These strategic initiatives help them improve sales, expand geographic reach, and enhance capacities to cater to a large customer base. A few of the significant developments in the cell and gene therapy market are; In September 2023, Agilent Technologies Inc. signed a Memorandum of Understanding (MOU) with the Advanced Cell Therapy and Research Institute, Singapore (ACTRIS). This agreement aims to improve cell and gene therapy advancements over the next three years. In March 2023, Cellevolve Bio partnered with Seattle Children's Therapeutics to develop and commercialize new multiplex CARs for pediatric cancers. With this collaboration, the companies will focus on the BrainChild research program-a suite of five multiplex CARs-to treat pediatric central nervous system (CNS) malignancies. They aim to leverage the Seattle Children's Cure Factory facility to conduct early clinical GMP research on new CARs. In March 2023, Twist Bioscience Corporation and Kriya Therapeutics, Inc. entered an antibody discovery agreement for antibodies delivered using adeno-associated viral (AAV) gene therapy in therapeutic oncology applications. Also, the companies aim to combine Twist's antibody libraries with Kriya's proprietary vector engineering platform to discover novel antibodies against specific targets of interest to be delivered with Kriya's gene therapy technology. In January 2023, FUJIFILM Corporation agreed to acquire a cell therapy manufacturing facility from Atara Biotherapeutics, Inc. The facility will witness expansion with the flexibility to produce clinical as well as commercial cell therapies, such as allogeneic T-cell and CAR T immunotherapies. The initiation of new businesses to remain competitive in the market through collaborations and partnerships can help accelerate the development of new platforms for cell and gene therapy manufacturing services. Thus, these strategic initiatives by key players are anticipated to create substantial growth opportunities in the Asia Pacific cell and gene therapy market during the forecast period.
Asia Pacific Cell and Gene Therapy Market Overview
China is making progress in gene and cell therapy. The country has a large patient population making it an attractive market for companies developing these treatments. Several companies are working on innovative therapies for diseases such as cancer and genetic disorders in China, and the country has invested heavily in gene therapy research and development. China was the first country to approve gene therapy in 2003; since then, cell and gene therapy developments have advanced rapidly worldwide, and their therapeutic potential has soared. The government of the People's Republic of China has conducted several regulatory reforms to promote the normative development of cell and gene therapies. According to a study published in Nature in 2021, China is home to more than 50% of all cell therapy trials worldwide. China has nearly 400 ongoing CAR-T trials centered on hematology, oncology, and solid tumors. Cell therapy in China is entering a new era with the approval of Fosun Kite's Yescarta and JW Therapeutics' Relma-cel in 2021 from the National Medical Products Administration (NMPA). In October 2023, CARsgen and IASO Biotherapeutics/Innovent were approved by the NMPA for new drug approvals targeting B-cell maturation antigens in CAR-T. These therapies are expected to be approved and commercialized soon.
Asia Pacific Cell and Gene Therapy Market Revenue and Forecast to 2031 (US$ Million)
Asia Pacific Cell and Gene Therapy Market Segmentation
The Asia Pacific cell and gene therapy market is categorized into type, services, scale, end user, and country.
Based on type, the Asia Pacific cell and gene therapy market is bifurcated into cell therapy and gene therapy. The cell therapy segment held a larger market share in 2023. Furthermore, the cell therapy segment is sub segmented into allogeneic, autologous, viral vectors. Additionally, the gene therapy segment is divided into non-viral vectors and viral vectors.
In terms of services, the Asia Pacific cell and gene therapy market is categorized process development, cGMP manufacturing, regulatory services, and bioassay services. The process development segment held the largest market share in 2023.
By scale, the Asia Pacific cell and gene therapy market is bifurcated into pre commercial/ R and D manufacturing and commercial scale manufacturing. The pre commercial/ R and D manufacturing segment held a larger market share in 2023.
Based on end user, the Asia Pacific cell and gene therapy market is segmented into contract research organizations, pharmaceutical and biopharmaceutical companies, and academic and research institutes. The pharmaceutical and biopharmaceutical companies segment held the largest market share in 2023.
By country, the Asia Pacific cell and gene therapy market is segmented into China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. China dominated the Asia Pacific cell and gene therapy market share in 2023.
Catalent Inc, Charles River Laboratories International Inc, F. Hoffmann-La Roche Ltd, FUJIFILM Holdings Corp, Lonza Group AG, Lotte Corp, Merck KGaA, Takara Bio Inc, Thermo Fisher Scientific Inc., and WuXi AppTec Co Ltd are some of the leading companies operating in the Asia Pacific cell and gene therapy market.

| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 1,024.37 Million |
| Market Size by 2031 | US$ 8,012.75 Million |
| CAGR (2023 - 2031) | 29.3% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
Asia Pacific
|
| Market leaders and key company profiles |
|

The Asia Pacific Cell and Gene Therapy Market is valued at US$ 1,024.37 Million in 2023, it is projected to reach US$ 8,012.75 Million by 2031.
As per our report Asia Pacific Cell and Gene Therapy Market, the market size is valued at US$ 1,024.37 Million in 2023, projecting it to reach US$ 8,012.75 Million by 2031. This translates to a CAGR of approximately 29.3% during the forecast period.
The Asia Pacific Cell and Gene Therapy Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Cell and Gene Therapy Market report:
The Asia Pacific Cell and Gene Therapy Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Cell and Gene Therapy Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Cell and Gene Therapy Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)