North American market for acute lung injury consists of the US, Canada, and Mexico. The US is the largest market for acute lung injury, followed by Canada and Mexico. The market across the US is expected to grow due to the well-established infrastructure, increasing prevalence of Acute Lung Injury and other lung diseases. Lung diseases like chronic obstructive pulmonary disease (COPD) and acute lung injury are the third leading cause of death in the US. Pneumonia is the direct cause of acute lung injury. According to the American Thoracic Society (ATS), pneumonia is the most common cause of sepsis and shock, causing 50% of all episodes. Pneumonia is a very common reason for US children being hospitalized. Every year, almost 1 million individuals in the United States seek hospital treatment for pneumonia, and 50,000 people die as a result of the illness. Also, according to National Hospital Ambulatory Medical Care Survey: 2018, the Annual number and percent distribution of emergency department visits by the Pneumonia diagnosis group in the United States was 1485. Market players are adopting organic and inorganic growth strategies for market development. For instance, in September 2020, Stemedica Cell Technologies, Inc. received US FDA investigational new drug (IND) approval for Phase II, controlled study for safety, tolerability, and efficacy assessment of intravenous allogeneic mesenchymal stem cells in patients with acute to severe lung injury due to COVID-19. Thus, owing to the factors mentioned above, it is expected that the acute lung injury market is likely to propel exponentially during the forecast years.
In case of COVID-19, North America is highly affected especially the US. The high number of COVID cases have resulted in a negative impact on country’s and region’s economy and there has been a decline in overall business activities and growth of various industries operating in the region. Market players are initiated clinical trials for the drug development of acute lung injury. For instance, Chimerix, a biopharmaceutical company initiated a Phase II/III clinical trial in June 2020 for to evaluate the safety and efficacy of Dociparstat sodium (DSTAT) in patients with Acute Lung Injury (ALI) due to COVID-19. DSTAT is a glycosaminoglycan derivative of heparin with robust anti-inflammatory properties. Chimerix completes enrolment in its phase II part of phase II/III trial for acute lung injury. The Phase 2 portion of the study will enroll 24 subjects to confirm the maximum safe dose and then expand it to 50 patients at the selected dose. Contingent upon positive results, the Phase 3 portion of the study will enroll approximately 450 subjects. Furthermore, Altasciences is pleased to support ReAlta Life Sciences by conducting a Phase I trial to evaluate RLS-0071 for treatment of Acute Lung Injury (ALI) as a result of viral infections, such as COVID-19, respiratory syncytial virus (RSV), and influenza. As part of the ReAlta ALI program underway, the Phase I trial is a single-ascending dose, randomized, double-blind, placebo-controlled, adaptive-design study to evaluate the safety, tolerability, PK, and PD of RLS-0071 in healthy subjects, performed at the Altasciences clinical pharmacology unit in Montreal, Canada.

Strategic insights for the North America Acute Lung Injury provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
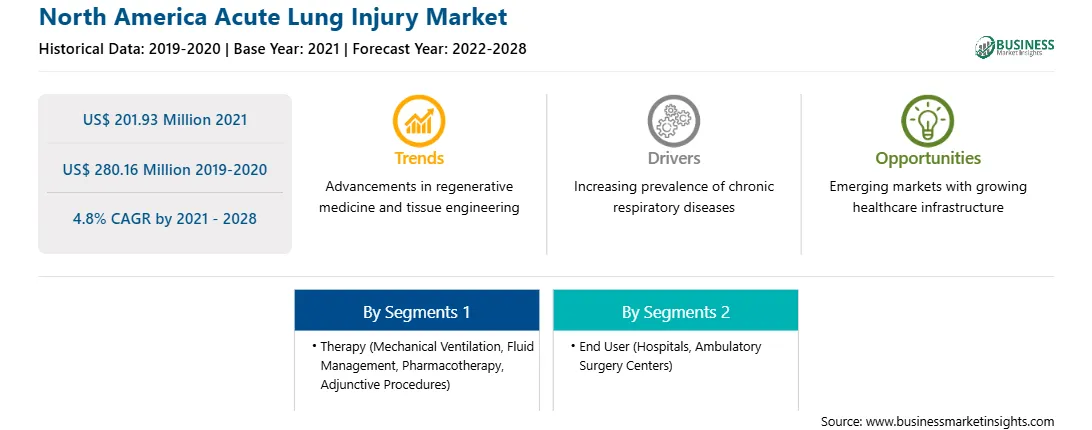
| Market size in 2021 | US$ 201.93 Million |
| Market Size by 2028 | US$ 280.16 Million |
| CAGR (2021 - 2028) | 4.8% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Therapy
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
|
The geographic scope of the North America Acute Lung Injury refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The North America acute lung injury market is expected to grow from US$ 201.93 million in 2021 to US$ 280.16 million by 2028; it is estimated to grow at a CAGR of 4.8% from 2021 to 2028. Manufacturers in the acute lung injury treatment market are focusing on the adoption of various strategies such as product innovation, product launches, and approvals, as well as R&D investment for advancements, along with inorganic strategies such as merger and acquisition and partnerships as their developmental strategies to maintain the competitive environment in the market. For instance, Faron Pharmaceuticals, Ltd. Is engaged in developing pharmacological treatments for ALI with the help of a consortium. The FP-1201-lyo treatment for acute lung injury is in the third phase of clinical trials and is anticipated to obtain marketing authorization during the forecast period. Similarly, in November 2020, Novartis entered into an exclusive license with Mesoblast to manufacture, develop, and commercialize remestemcel-L to treat ARDS associated with COVID-19. Moreover, recent advances in understanding ALI pathophysiology have led to investigations of numerous potential pharmacologic treatments. Therefore, the introduction of advanced products is likely to revolutionize and offer opportunities for the growth of the North America acute lung injury market.
In terms of therapy, the mechanical ventilation segment accounted for the largest share of the North America acute lung injury market in 2020. In terms of end user, the hospitals segment held a larger market share of the North America acute lung injury market in 2020.
A few major primary and secondary sources referred to for preparing this report on the North America acute lung injury market are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Angion; Asklepion Pharmaceuticals, LLC.; GlaxoSmithKline Plc; Qx Therapeutics, Inc.; ReAlta Life Sciences, Inc.; Stemedica Cell Technologies, Inc; and Windtree Therapeutics, Inc.
The North America Acute Lung Injury Market is valued at US$ 201.93 Million in 2021, it is projected to reach US$ 280.16 Million by 2028.
As per our report North America Acute Lung Injury Market, the market size is valued at US$ 201.93 Million in 2021, projecting it to reach US$ 280.16 Million by 2028. This translates to a CAGR of approximately 4.8% during the forecast period.
The North America Acute Lung Injury Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Acute Lung Injury Market report:
The North America Acute Lung Injury Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Acute Lung Injury Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Acute Lung Injury Market value chain can benefit from the information contained in a comprehensive market report.