The European medical devices and diagnostics contract research organization market is divided into Germany, UK, France, Italy, Spain, and Rest of Europe. Europe occupies a significant position in the global medical devices and diagnostics contract research organization market and is estimated to register a robust growth rate over the forecast period. The market growth in the region is expected due to the increasing demand for medical devices, growing research, and development in the field of medical technology, rising numbers of hospitals that are dedicated geriatric population. Likewise, rising funding for medical device innovation and the emergence of breakthrough discoveries in Medtech and medical robotics is likely to support the market growth during the forecast period.
Europe is highly affected by the COVID-19 pandemic. Countries such as Italy, Spain, the UK, Germany, France, Russia, and Switzerland are highly affected. Most of the deaths were registered in these countries in the initial phases of the pandemic onset. The EU countries even faced the second wave of the pandemic. Therefore, to control the spread of COVID-19, governments of various countries have implemented lockdown. The MedTech industry and its organizations recently called for the postponement of the implementation of the EU MDR and EU IVDR because of the COVID-19 pandemic. Various regulatory changes were scheduled to come into effect in May 2022 as part of the new EU IVDR; however, the dates are likely to be delayed a year because of the COVID-19 pandemic. According to the Greenlight Guru 2021 medical device industry benchmark study fielded in October 2020, 63% of industry professionals feel the pandemic negatively affected funding at their organization, 39% found COVID-19 harming financial viability, and another 59% stated it had negatively affected time to market.
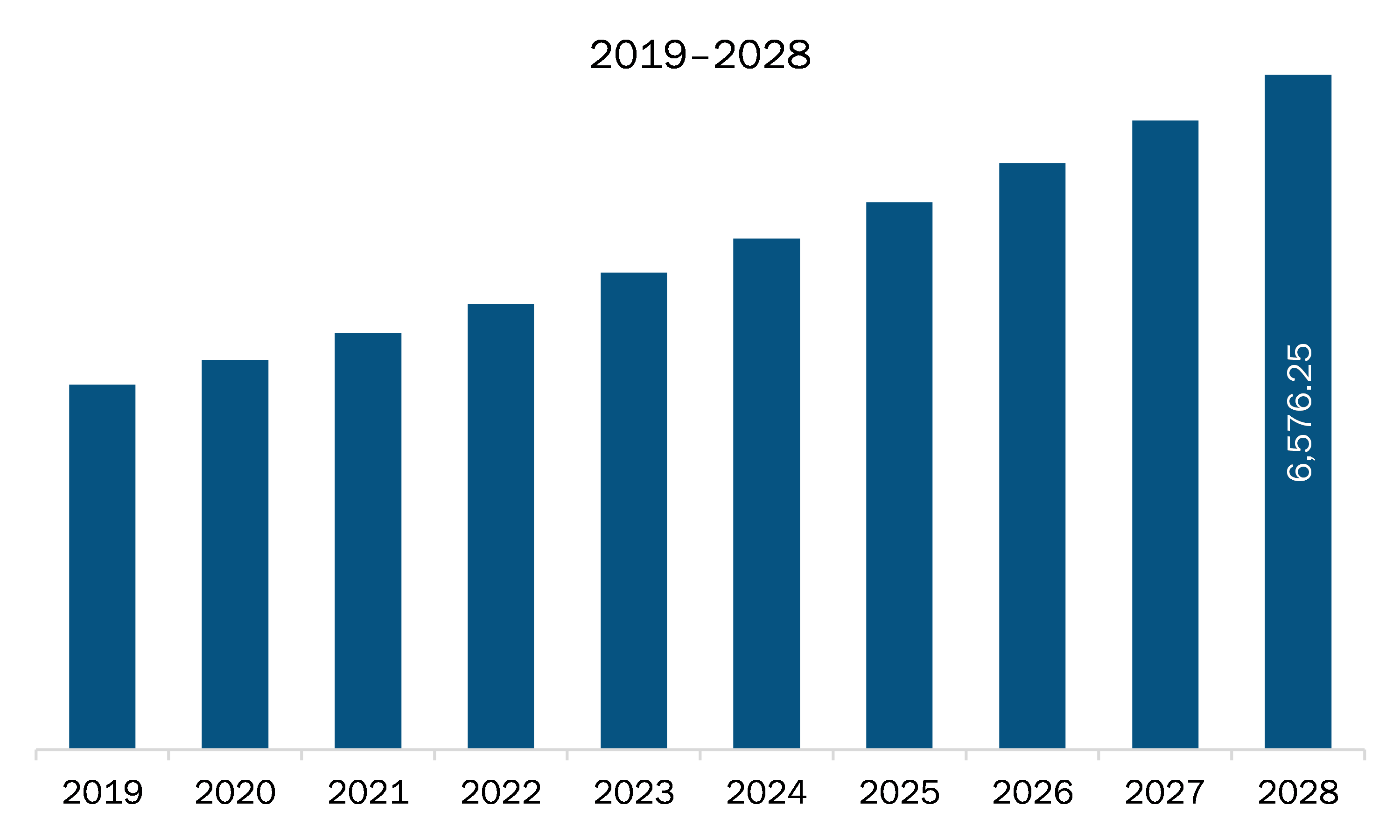
Strategic insights for the Europe Medical Device and Diagnostics Contract Research Organization provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
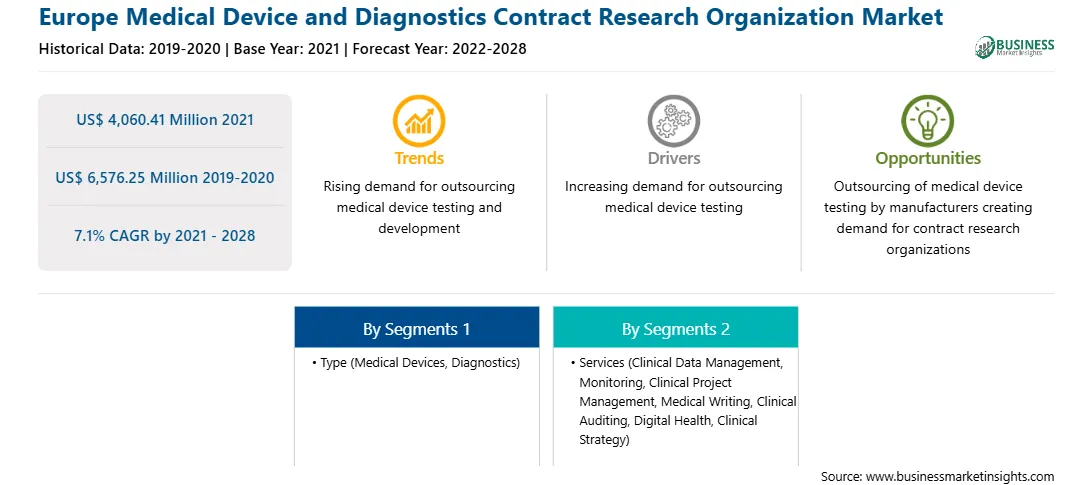
| Market size in 2021 | US$ 4,060.41 Million |
| Market Size by 2028 | US$ 6,576.25 Million |
| CAGR (2021 - 2028) | 7.1% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
|
The geographic scope of the Europe Medical Device and Diagnostics Contract Research Organization refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The medical device and diagnostics contract research organization market in Europe is expected to grow from US$ 4,060.41 million in 2021 to US$ 6,576.25 million by 2028; it is estimated to grow at a CAGR of 7.1% from 2021 to 2028. Clinical trial is a crucial and significant step to evaluate the safety and effectiveness of a medical strategy, treatment, or device for commercial usage. These studies also help understand and determine the best medical approaches for a particular therapeutic area. Clinical trials are conducted primarily to collect data regarding the safety and efficacy of new drug and device development. Before the approval of drug molecules or medical devices by the regulatory authorities, a series of clinical studies are conducted. The increasing prevalence of various communicable and noncommunicable diseases is creating the need for the development of new drugs or medical devices, which is, in turn, propelling the demand for the medical device & diagnostics contract research organization. Hence, the increasing number of clinical trials accelerates the demand for medical device & diagnostics contract research organization, which drives the growth of the medical device & diagnostics contract research organization market.
The Europe medical device and diagnostics contract research organization market is segmented into type, services, and country. Based on type, the Europe medical device and diagnostics contract research organization market is segmented into medical devices and diagnostic. Diagnostic segment is further sub-segmented into cardiac biomarkers, diabetes management, oncology, infectious diseases, hematology, chemistry and immunoassays, molecular diagnostics, and others. The medical devices segment held the largest share of the market in 2020. Based on services, the Europe medical device and diagnostics contract research organization market is segmented into clinical data management, monitoring, clinical project management, medical writing, clinical auditing, digital health, clinical strategy, and others. The clinical data management segment held the largest share of the market in 2020. Based on country Europe medical device and diagnostics contract research organization market is segmented into France, Spain, Germany, Italy, and UK.
A few major primary and secondary sources referred to for preparing this report on the medical device and diagnostics contract research organization market in Europe are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Activa CRO; Charles River Laboratories, Inc; ICON PLC; IQVIA; Laboratory Corporation of America Holdings; North American Science Associates, Inc.; PAREXEL International Corporation; Qserve Group B.V.; and WUXI APPTEC.
The Europe Medical Device and Diagnostics Contract Research Organization Market is valued at US$ 4,060.41 Million in 2021, it is projected to reach US$ 6,576.25 Million by 2028.
As per our report Europe Medical Device and Diagnostics Contract Research Organization Market, the market size is valued at US$ 4,060.41 Million in 2021, projecting it to reach US$ 6,576.25 Million by 2028. This translates to a CAGR of approximately 7.1% during the forecast period.
The Europe Medical Device and Diagnostics Contract Research Organization Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Medical Device and Diagnostics Contract Research Organization Market report:
The Europe Medical Device and Diagnostics Contract Research Organization Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Medical Device and Diagnostics Contract Research Organization Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Medical Device and Diagnostics Contract Research Organization Market value chain can benefit from the information contained in a comprehensive market report.