Asia Pacific Medical Device and Diagnostics Contract Research Organization Market
No. of Pages: 139 | Report Code: TIPRE00024092 | Category: Life Sciences
No. of Pages: 139 | Report Code: TIPRE00024092 | Category: Life Sciences
APAC medical devices and diagnostics contract research organization market is analyzed based on the five major countries such as China, Japan, India, Australia, South Korea, and the rest of APAC. China is expected to dominate the medical devices and diagnostics contract research organization market in APAC. The market in China is expected to grow due to factors such as high demand and export of medical supplies and devices, large population leading to high number of patients suffering with various disease requiring a broad range of medical devices for diagnostics and therapeutics.
Countries in APAC are facing an increasing incidence of COVID-19. Accompanying the catastrophic loss of life that the virus has caused is the impact on the global economy, which has reeled from the effects of the pandemic. The COVID-19 pandemic has critically impacted the region; however, it has also provided an opportunity for stakeholders to realign their future path based on strategic intent and focus. COVID-19 has created an extraordinary emergency that is particularly affecting the supply chain. The supply chain disruptions, along with the enormous need for medical devices and protective health care material, have generated the need for new initiatives and the use of emerging technologies such as three-dimensional printing (3DP) to come forward and support health care professionals and supply chain. The COVID-19 pandemic has adversely impacted the region, but it has also provided an opportunity for stakeholders to realign their future path based on strategic intent and focus. India and China are the most preferred destinations for outsourcing, where a large pool of skilled labor is available at a lower cost. With increasing pressure to reduce manufacturing costs and accelerate time-to-market, many devices and diagnostics companies are looking to conduct clinical trial operations in APAC. Increasingly, third-party outsourcing service providers are playing a greater role in supporting and managing the delivery of key business functions like medical device development, sales and marketing, and regulatory compliance services in APAC.
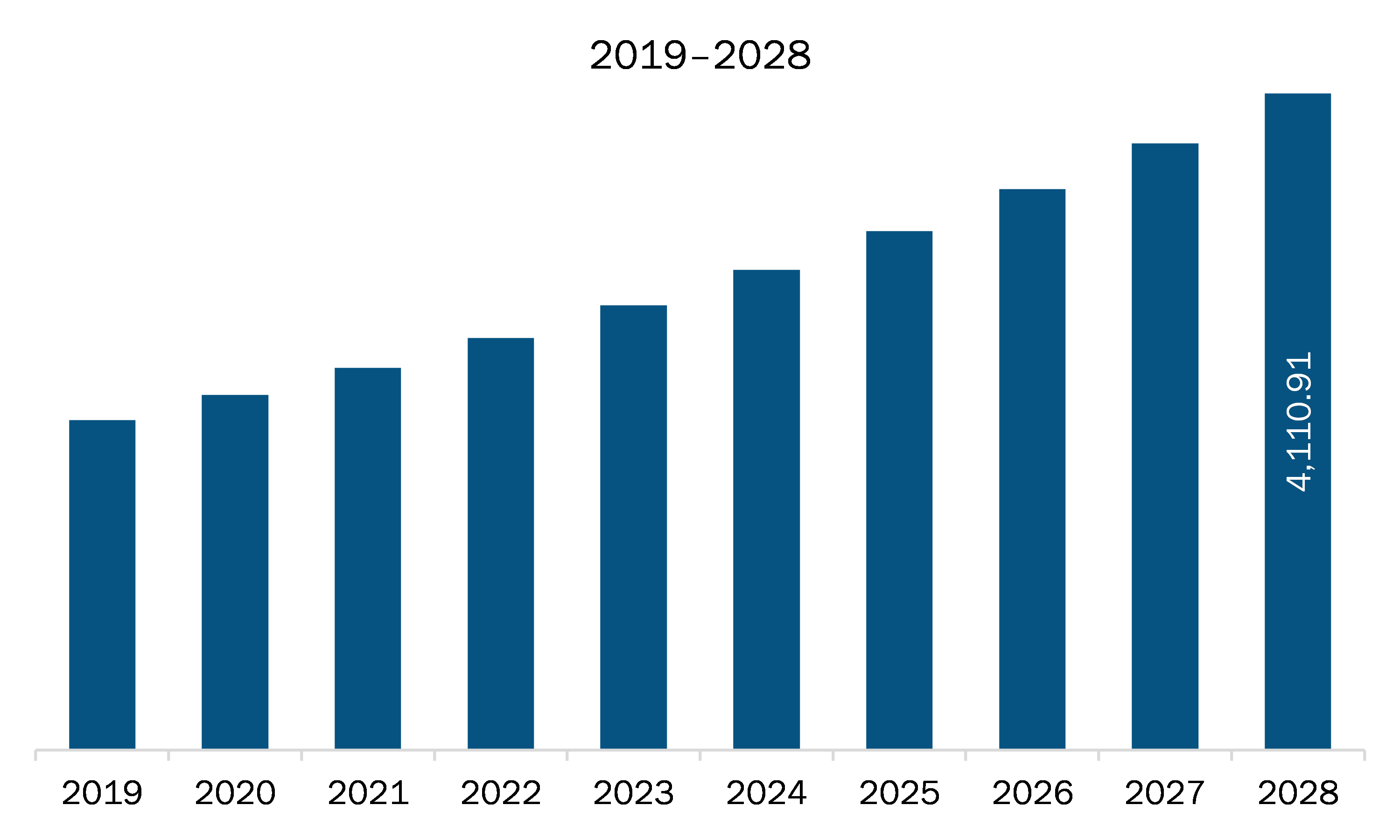
Strategic insights for the Asia Pacific Medical Device and Diagnostics Contract Research Organization provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
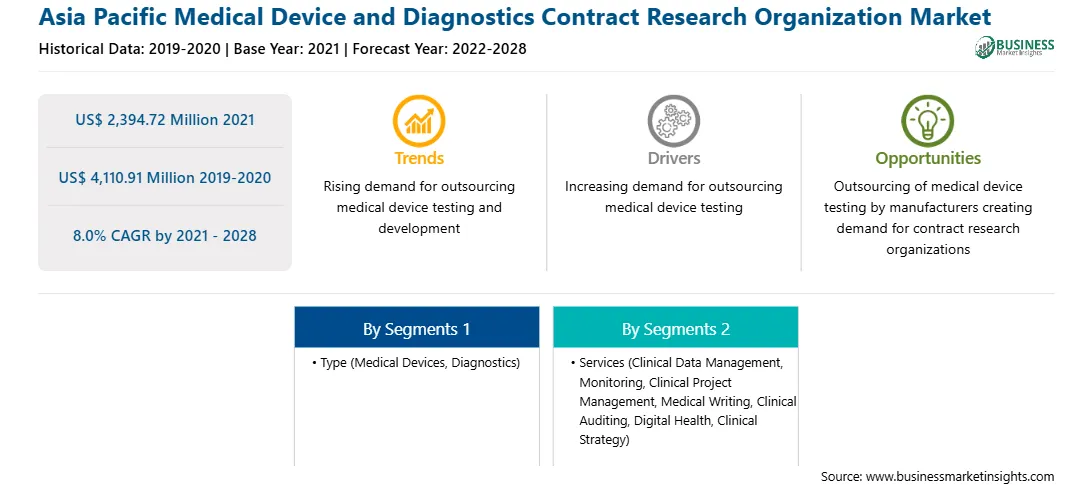
| Market size in 2021 | US$ 2,394.72 Million |
| Market Size by 2028 | US$ 4,110.91 Million |
| CAGR (2021 - 2028) | 8.0% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
|
The geographic scope of the Asia Pacific Medical Device and Diagnostics Contract Research Organization refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The medical device and diagnostics contract research organization market in APAC is expected to grow from US$ 2,394.72 million in 2021 to US$ 4,110.91 million by 2028; it is estimated to grow at a CAGR of 8.0% from 2021 to 2028. The drug development and discovery are a time consuming and expensive process. The process from early discovery or design to development to regulatory approval takes more than 10 to 15 years. Throughout the development phase of a drug substance, various testing services are required to check the quality and efficacy of the product. Therefore, the pharmaceutical and biotechnology companies prefer to outsource the services from the medical device & diagnostics contract research organization to save the cost and time, which boosts the growth of the market.
The APAC medical device and diagnostics contract research organization market is segmented into type, services, and country. Based on type, the APAC medical device and diagnostics contract research organization market is segmented into medical devices and diagnostic. Diagnostic segment is further sub-segmented into cardiac biomarkers, diabetes management, oncology, infectious diseases, hematology, chemistry and immunoassays, molecular diagnostics, and others. The medical devices segment held the largest share of the market in 2020. Based on services, the APAC medical device and diagnostics contract research organization market is segmented into clinical data management, monitoring, clinical project management, medical writing, clinical auditing, digital health, clinical strategy, and others. The clinical data management segment held the largest share of the market in 2020. Based on country APAC medical device and diagnostics contract research organization market is segmented into Australia, China, India, Japan, and South Korea.
A few major primary and secondary sources referred to for preparing this report on the medical device and diagnostics contract research organization market in APAC are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Charles River Laboratories, Inc; ICON PLC; IQVIA; Laboratory Corporation of America Holdings; APACn Science Associates, Inc.; PAREXEL International Corporation; Qserve Group B.V.; and WUXI APPTEC.
The Asia Pacific Medical Device and Diagnostics Contract Research Organization Market is valued at US$ 2,394.72 Million in 2021, it is projected to reach US$ 4,110.91 Million by 2028.
As per our report Asia Pacific Medical Device and Diagnostics Contract Research Organization Market, the market size is valued at US$ 2,394.72 Million in 2021, projecting it to reach US$ 4,110.91 Million by 2028. This translates to a CAGR of approximately 8.0% during the forecast period.
The Asia Pacific Medical Device and Diagnostics Contract Research Organization Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Medical Device and Diagnostics Contract Research Organization Market report:
The Asia Pacific Medical Device and Diagnostics Contract Research Organization Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Medical Device and Diagnostics Contract Research Organization Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Medical Device and Diagnostics Contract Research Organization Market value chain can benefit from the information contained in a comprehensive market report.