The Asia Pacific therapeutic vaccines market size is expected to reach US$ 85,862.88 thousand by 2031 from US$ 34,213.87 thousand in 2024. The market is estimated to record a CAGR of 14.0% from 2024–2031.
The Asia Pacific therapeutic vaccine market is experiencing significant growth, driven by technological advancements and increased investments in vaccine research. China and India are key contributors to this growth due to their large populations, robust immunization initiatives, and rising government spending on healthcare. Additionally, Japan's advanced healthcare system and high vaccination coverage significantly enhance market expansion. This region is also seeing rapid growth, thanks to an increase in public health programs and improved healthcare infrastructure. In Australia, strong government support and high public awareness of vaccination benefits further bolster the market. However, the market faces several challenges, including stringent regulatory requirements, high production and development costs, and low awareness levels among patients and healthcare providers. Addressing these issues is essential for the sustained growth of the therapeutic vaccine market in the Asia Pacific region.
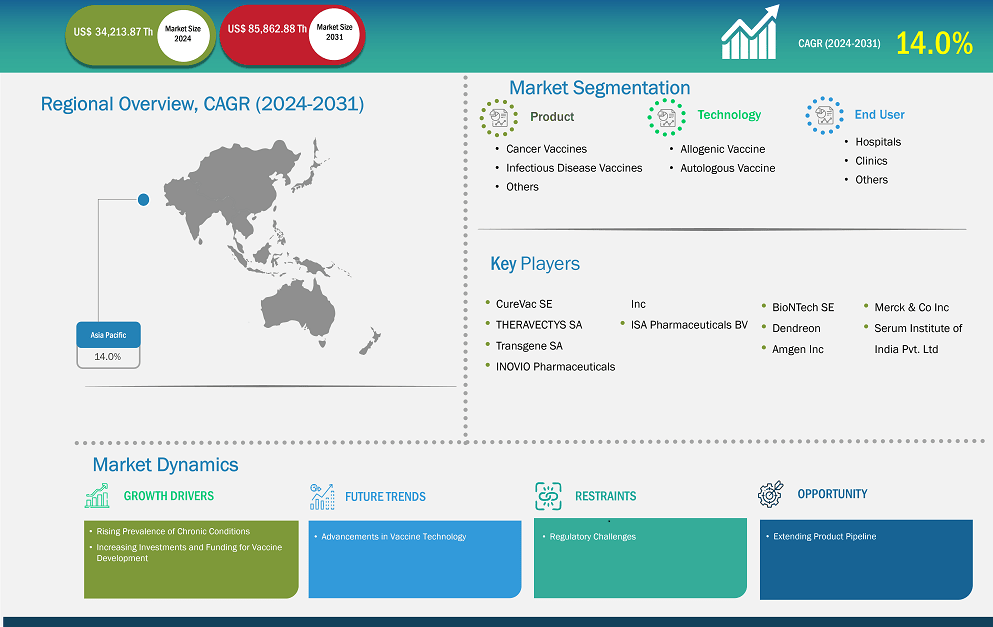
Key segments that contributed to the derivation of the therapeutic vaccines market analysis are product, technology, and end user.
As the global demand for innovative treatment options rises, the therapeutic vaccines sector has witnessed robust investments and advancements in vaccine development, driven largely by the rising number of clinical candidates progressing through various stages of development. Innovative R&D efforts in biotechnology are yielding cutting-edge therapeutic vaccines that target various diseases, particularly newer infections and chronic ones such as cancer. Moderna's mRNA-4157 is currently in phase 2 trials, with early-stage results showing promising potential in combination with checkpoint inhibitors for melanoma treatment. In addition to oncology, the therapeutic vaccine pipeline is also growing in areas such as autoimmune diseases and infectious diseases (HIV, HPV, and HBV infections).
Several companies are investing in scientific and technical excellence to develop and introduce new therapeutic vaccines. With the expanding pipeline of vaccine candidates targeting diverse diseases, therapeutic vaccines hold immense potential to address unmet medical needs. Below is the product pipeline for therapeutic vaccines targeting various conditions:
|
Vaccine type |
Vaccine Formulation |
Phase/status |
Condition |
|
Bacterial vector vaccine |
ADXS11–001 (attenuated live Listeria Encoding HPV 16 E7 vector) |
Phase I/II |
Cervical carcinoma Cervical cancer Anal cancer Rectal cancer |
|
Peptide/protein vaccine |
ISA101 (nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with Montanide ISA51) |
Phase II |
Solid tumors |
|
Personalized peptide vaccine |
NeoVax (neoantigen + Poly-ICLC) |
Phase I |
Kidney cancer |
|
mRNA-based neoantigen vaccine |
mRNA-4157 (lipid-encapsulated RNA) |
Phase I/II |
Solid tumors Melanoma |
|
DNA vaccine |
VGX-3100 (plasmid encoding E6 and E7 of HPV16/18) |
Phase I/II |
Head and neck squamous cell cancer Cervical cancer |
|
Virus-like particle |
NASVAC (HBsAg, HBcAg) |
Phase III |
HBV infection |
|
Protein vaccine |
Theravax (DV-601, consisting of HBsAg, HbcAg, and saponin-based ISCOMATRIX adjuvant) |
Phase II |
Chronic HBV infection |
|
mRNA vaccine |
iHIVARNA (consisting of HIV immunogen sequence and a mixture of activation molecules) |
Phase I |
HIV infection |
|
Viral vector vaccines |
AdCh3NSmut/Ad6NSmut (encoding HCV proteins) TG4040 (expressing NS3/4/5B proteins) |
Phase I |
HCV infection |
|
DNA vaccine |
pNGVL4a-Sig/E7(detox)/HSP70 (HPV16 E7) |
Phase I |
HPV infection |
With strong ongoing clinical trials, the market's prospects are expected to expand, offering new treatment options for patients worldwide and addressing unmet medical needs in areas where current treatments are ineffective. Thus, a strong pipeline of candidates for therapeutic vaccines signifies lucrative growth opportunities for the therapeutic vaccines market.
Based on country, the Asia Pacific therapeutic vaccines market comprises the China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. Australia held the largest share in 2024.
The therapeutic vaccines market in Australia is poised for growth due to the country's robust healthcare infrastructure and active participation in vaccine development efforts. In August 2023, Australian researchers made noteworthy strides in developing a highly effective vaccine targeting EBV, which markets a significant event in the context of therapeutic vaccines. This new EBV vaccine represents a promising advancement in therapeutic strategies, particularly for conditions linked to EBV, such as multiple sclerosis (MS) and cancer indications. The development of a therapeutic EBV vaccine could transform the management of diseases associated with EBV. By effectively targeting the virus and its effects on the immune system, this vaccine has the potential to not only treat existing infections but also to mitigate the long-term health risks associated with the virus. As research progresses, this vaccine could pave the way for new treatment protocols that enhance patient outcomes and reduce the burden of EBV-related diseases.
South Australia is set to establish itself as a leader in mRNA technology with the development of a state-of-the-art mRNA development and production facility. The BioCina facility will serve as a center of excellence for mRNA production, focusing on the scale-up and manufacturing of therapeutic drugs and vaccines. In the future, this facility is expected to contribute to international efforts in combating diseases through innovative mRNA therapies in addition to supporting local health initiatives. BioCina has secured US$ 10 million in funding, with equal contributions from the Australian Government's National Health and Medical Research Council and the Government of South Australia. With this funding, it would streamline the production of therapeutic drugs and vaccines within the next 2 years of receiving the amount, transforming the facility into an mRNA Centre of Excellence. In May 2023, the University of Adelaide's School of Chemical Engineering played a pivotal role in developing cutting-edge technology aimed at creating and producing personalized mRNA therapeutic drugs and vaccines in Adelaide. The university takes great pride in its ongoing collaboration with BioCina, which exemplifies the successful collaboration between universities and industry to achieve mutually beneficial outcomes.
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 34,213.87 Thousand |
| Market Size by 2031 | US$ 85,862.88 Thousand |
| CAGR (2024 - 2031) | 14.0% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
Asia Pacific
|
| Market leaders and key company profiles |
|
Some of the key players operating in the market include CureVac SE, THERAVECTYS SA, Transgene SA, INOVIO Pharmaceuticals Inc, ISA Pharmaceuticals BV, Amgen Inc, Merck & Co Inc, Serum Institute of India Pvt. Ltd, BioNTech SE, and Dendreon among others. These players are adopting various strategies such as expansion, product innovation, and mergers and acquisitions to provide innovative products to their consumers and increase their market share.
The following methodology has been followed for the collection and analysis of data presented in this report:
The research process begins with comprehensive secondary research, utilizing both internal and external sources to gather qualitative and quantitative data for each market. Commonly referenced secondary research sources include, but are not limited to:
Note: All financial data included in the Company Profiles section has been standardized to USD. For companies reporting in other currencies, figures have been converted to USD using the relevant exchange rates for the corresponding year.
The Insight Partners conducts a significant number of primary interviews each year with industry stakeholders and experts to validate its data analysis, and gain valuable insights. These research interviews are designed to:
Primary research is conducted via email interactions and telephone interviews, encompassing various markets, categories, segments, and sub-segments across different regions. Participants typically include:
The Asia Pacific Therapeutic Vaccines Market is valued at US$ 34,213.87 Thousand in 2024, it is projected to reach US$ 85,862.88 Thousand by 2031.
As per our report Asia Pacific Therapeutic Vaccines Market, the market size is valued at US$ 34,213.87 Thousand in 2024, projecting it to reach US$ 85,862.88 Thousand by 2031. This translates to a CAGR of approximately 14.0% during the forecast period.
The Asia Pacific Therapeutic Vaccines Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Therapeutic Vaccines Market report:
The Asia Pacific Therapeutic Vaccines Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Therapeutic Vaccines Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Therapeutic Vaccines Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)