The high presence of unmet medical needs for diseases like cancer, diabetes, cardiovascular and infectious diseases and increased awareness among the patients and physicians regarding early diagnosis of these diseases have driven the point of care diagnostics market in Asia Pacific. The prevalence of diabetes in Asia Pacific is on a constant rise, thus, generating a broad patient base for the point of care glucose testing segment. According to the International Diabetes Federation, in 2019, about 88 million adults were living with diabetes in the Southeast Asia (SEA) region, and more than half of people with diabetes (57%) in the SEA region were undiagnosed. Point-of-care testing (POCT) plays a critical role in diagnosing, treating, and preventing infectious diseases. POC testing can be used to detect several major pathogens, including malarial parasites, human immunodeficiency virus (HIV), human papillomavirus (HPV), dengue, Ebola, and Zika viruses, and Mycobacterium tuberculosis (TB bacteria). Moreover, healthcare-associated infections (HAIs) such as central line-associated bloodstream infections and catheter-associated urinary tract infections might affect the patients in hospitals and other healthcare facilities. According to a recent study published on NCBI in 2020, HAI causes around 16 million additional hospitalization days in Asia Pacific each year. Moreover, according to the same study, HAI leads to an additional financial burden of around US$ 8.5 billion each year. In addition, the COVID-19 pandemic has resulted in increasing adoption of point of care diagnostics, which is further driving the market.
The developing healthcare infrastructure in Asia Pacific is creating significant opportunities for the key market players to expand their business. This is likely to be a prime factor contributing to the market growth during the forecast period. Most of the major players focus on developing countries such as India and China, owing to a large population suffering from HIV and other infectious diseases. Moreover, the increasing number of product launches indicates a potential environment for adopting advanced point of care diagnostic kits, which offers a significant opportunity for market growth. For instance, in November 2020, Abbott Laboratories announced the launch of its FreeStyle Libre glucose monitoring system in India. The new device incorporates technology to continually measure blood glucose levels and delivers real-time measurements at any point in time.
In addition, various conferences and events are held to make people aware of devices and give an overview of the common key principles that should be applied when establishing and maintaining point of care testing (POCT) services. Participants in these conferences/events can utilize these learnings to develop a framework for introducing POCT for a specific clinical need relevant to their family practice. For instance, in November 2018, SELECTBIO organized the Asia Diagnostics Summit in Taiwan, focusing on point of care diagnostics, rapid and low-cost diagnostics for deployment worldwide, liquid biopsies for cancer, and the development of immunoassays for various analyte classes of relevance in diagnostics. Thus, increased demand for point of care diagnosis, owing to increased awareness and advancements in the healthcare setting in Asian countries, is anticipated to provide significant growth opportunities to the players operating in the Point-of-Care Diagnostic market during the forecast period.
Strategic insights for the Asia Pacific Point-of-Care Diagnostic provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
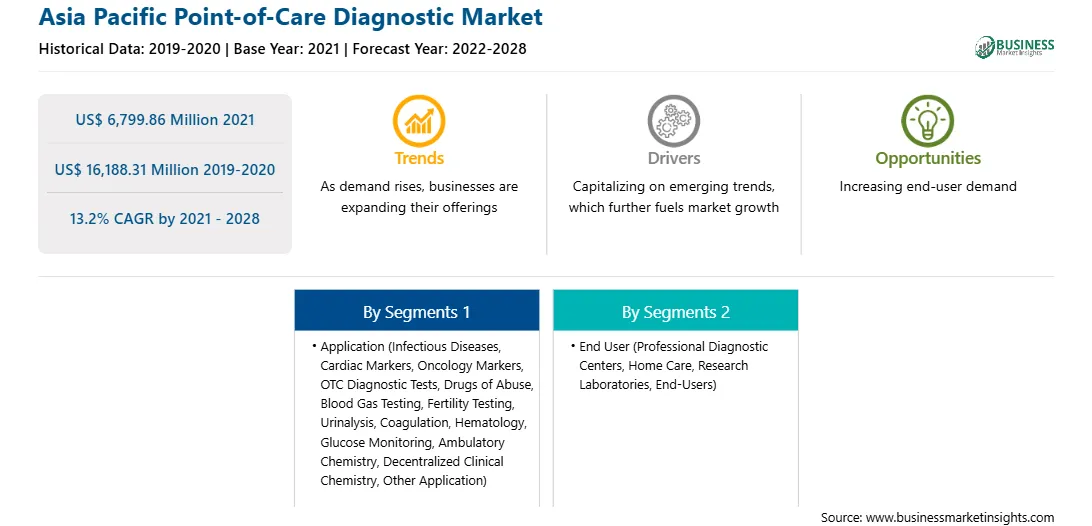
| Market size in 2021 | US$ 6,799.86 Million |
| Market Size by 2028 | US$ 16,188.31 Million |
| CAGR (2021 - 2028) | 13.2% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Application
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
|
The geographic scope of the Asia Pacific Point-of-Care Diagnostic refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
Based on application, the APAC point-of-care diagnostic market is segmented into infectious disease, cardiac markers, oncology markers, OTC diagnostic tests, drugs of abuse, blood gas testing, fertility testing, urinalysis, coagulation, hematology, glucose monitoring, ambulatory chemistry, decentralized clinical chemistry, and other applications. The glucose monitoring segment held the largest share of the market in 2021, whereas the infectious disease segment is expected to register the highest CAGR during the forecast period. The market growth for this segment is driven by the prevalence of infectious diseases, including COVID-19; the growing awareness for early disease diagnosis; shifting focus from centralized laboratories to decentralized POC testing; and rising technological advancements. For instance, in October 2020, Siemens Healthineers launched rapid antigen test for the detection of SARS-CoV-2. Such novel product launches would significantly augment the market growth for this segment during the forecast period.
Various business strategies such as collaborations, product innovations, and technology partnerships by the players in the Asia Pacific point-of-care diagnostic market bridge the demand–supply gap.
The Asia Pacific Point-of-Care Diagnostic Market is valued at US$ 6,799.86 Million in 2021, it is projected to reach US$ 16,188.31 Million by 2028.
As per our report Asia Pacific Point-of-Care Diagnostic Market, the market size is valued at US$ 6,799.86 Million in 2021, projecting it to reach US$ 16,188.31 Million by 2028. This translates to a CAGR of approximately 13.2% during the forecast period.
The Asia Pacific Point-of-Care Diagnostic Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Point-of-Care Diagnostic Market report:
The Asia Pacific Point-of-Care Diagnostic Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Point-of-Care Diagnostic Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Point-of-Care Diagnostic Market value chain can benefit from the information contained in a comprehensive market report.