Over the last decade, the pharmaceutical industry has witnessed many transformational trends and innovations that have the potential to improve medicines available on the market worldwide rapidly. In the last 10 years, the influence of AI and Big Data analytics on disease diagnosis and treatment and a shift toward preventing life-threatening conditions rather than curing them have led to the evolution of healthcare. With the continuous evolution of science and technology, exciting developments in the field of high throughput ADME (HT-ADME) research are highly anticipated in the next decade. The emerging fields of ADME sciences will find their way into ADME screening studies from the perspective of assay repertoire.
The novel, physiologically appropriate in-vitro micro-physiological systems (MPS), such as 3D tissue cultures and organ(s)-on-a-chip, are likely to make their way in the improvement efforts for in-vitro in-vivo translation (IVIVT). The use of endogenous probes (basic biomarkers) for in vivo transporter drug-drug interaction (DDI) investigations is coming up as another trend in ADME testing, as this approach can lower pill burden and help obtain transporter DDI data from a standard phase I trial. The HT-ADME screening of innovative drug delivery systems is yet another example. The field is prepared to undertake early ADME screening of novel modalities, such as protein degraders, antisense oligonucleotides (ASOs), antibody-drug conjugates (ADCs), and biologics, in addition to small molecules and peptides. Although the ADME studies for these modalities are still developing, an increasing number of HT-ADME format assays are anticipated to go online to support ongoing scientific research. With the long history of HT-ADME operation in many companies and the resulting wealth of ADME data that can be “mined,” the development of in silico models to predict ADME properties has long been acknowledged as the obvious next step for HT-ADME screening. The HT-ADME dataset of any big pharmaceutical company could include test findings from numerous compounds, in contrast to many ADME prediction models described in the literature that employ a training set of a smaller number of compounds. Due to their size, these datasets can be the best training sets for model building. ADME features have long been studied using computational chemistry methods designed to create quantitative structure-activity relationship (QSAR) models. Models for clearance, permeability, and DDI potentials have recently been developed using various machine learning (ML) techniques. Hit triaging from lead discovery screens and the design-make-test cycles of lead optimization in drug discovery, among others, can be employed to obtain predictive ADME models due to the recent rapid developments in ML methodologies and the large size of the HT-ADME datasets available for use as training sets. These top trends will transform the pharmaceutical industry and can have short-term and long-term benefits on the ADMET testing market.
Strategic insights for the Asia Pacific Pharma ADMET Testing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Asia Pacific Pharma ADMET Testing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Asia Pacific Pharma ADMET Testing Strategic Insights
Asia Pacific Pharma ADMET Testing Report Scope
Report Attribute
Details
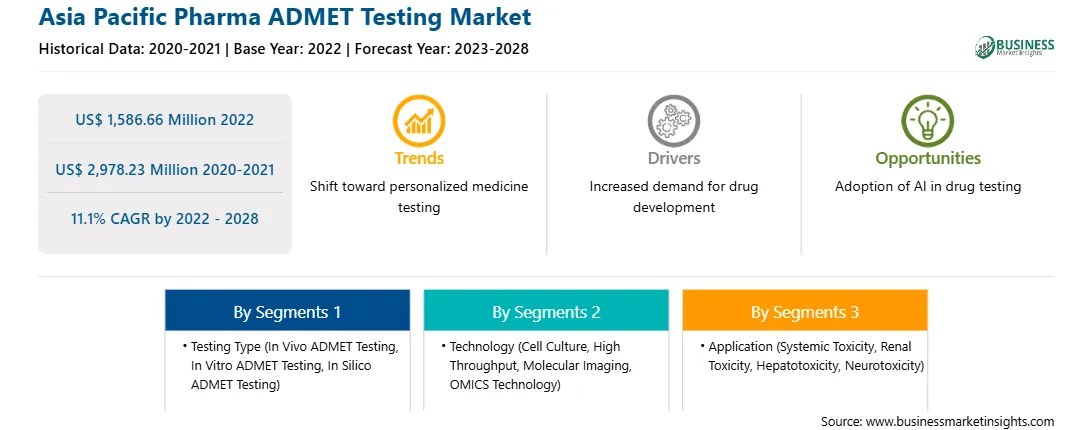
Market size in 2022
US$ 1,586.66 Million
Market Size by 2028
US$ 2,978.23 Million
CAGR (2022 - 2028) 11.1%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Testing Type
By Technology
By Application
Regions and Countries Covered
Asia-Pacific
Market leaders and key company profiles
Asia Pacific Pharma ADMET Testing Regional Insights
The Asia Pacific pharma ADMET testing market is segmented by testing type, technology, application, and country. Based on testing type, the market is segmented into in vivo ADMET testing, in vitro ADMET testing, and in silico ADMET testing. The in vivo ADMET testing segment is dominating the market in 2022. Based on technology, the market is segmented into cell culture, high throughput, molecular imaging, and OMICS technology. The cell culture segment is dominating the market in 2022. Based on application, the market is segmented into systemic toxicity, renal toxicity, hepatotoxicity, neurotoxicity, and others. The systemic toxicity segment is dominating the market in 2022. Based on country, the market is segmented into China, Japan, India, Australia, South Korea, and the rest of Asia Pacific. Further, China dominated the market in 2022.
A few key players dominating the Asia Pacific pharma ADMET testing market are Agilent Technologies, Inc.; Bio-Rad Laboratories, Inc.; Biovia (Dassault Systèmes); Charles River Laboratories; IQVIA Inc.; MERCK KGaA; Promega Corporation; and Wuxi AppTec.
The Asia Pacific Pharma ADMET Testing Market is valued at US$ 1,586.66 Million in 2022, it is projected to reach US$ 2,978.23 Million by 2028.
As per our report Asia Pacific Pharma ADMET Testing Market, the market size is valued at US$ 1,586.66 Million in 2022, projecting it to reach US$ 2,978.23 Million by 2028. This translates to a CAGR of approximately 11.1% during the forecast period.
The Asia Pacific Pharma ADMET Testing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Pharma ADMET Testing Market report:
The Asia Pacific Pharma ADMET Testing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Pharma ADMET Testing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Pharma ADMET Testing Market value chain can benefit from the information contained in a comprehensive market report.