The US Helicobacter Pylori non-invasive testing market growth is attributed to the increasing prevalence of H. pylori bacterial infection and rising market development activities in H. pylori non-invasive test. However, asymptomatic nature of H. pylori is hampering the growth of the US Helicobacter Pylori Non-Invasive Testing market.
H. pylori is a bacterium causing stomach infection, peptic ulcer, and gastritis. The prolonged bacterial infection may lead to stomach cancer in some cases. The H. pylori infection is more common in the US Hispanic and black populations, which can be credited to socioeconomic factors such as low income and literacy, household crowding, and large-scale immigration. Thus, increase in prevalence of H. pylori infection fuel the US Helicobacter pylori non-invasive testing market.
The H. pylori infection usually occurs in children with long-standing clinical symptoms such as gastritis, peptic ulcer disease, and stomach cancer. According to an article published in the Nature Journal, H. pylori is present in ~36% of the US population, posing a significant risk for gastric cancer and peptic ulcer disease. The World Health Organization (WHO) has recognized H. pylori as a carcinogen that leads to gastric cancer and in the US, gastric cancer is the third most common gastrointestinal cancer. According to the American Cancer Society, nearly 26,500 new cases of stomach cancer and ~11,130 deaths will occur due to this cancer type in 2023. Thus, growing cancer cases increases the US Helicobacter pylori non-invasive testing market growth.
Further, the growing geriatric population pool susceptible to gastric diseases will substantially increase the risk of H. pylori infection. Aging leads to thinning of the stomach lining, thus allowing the bacteria to pass through the protective lining, thereby increasing the disease prevalence among older people. Given the constant increase in the geriatric population base, the Washington Post has estimated that there will be nearly 80.8 million senior citizens in the US by 2040. Thus, growing geriatric population boosts the growth of US Helicobacter pylori non-invasive testing market.
According to a report from the National Institutes of Health, there was a 5% prevalence of H. pylori in children under the age of 10 in the US in 2020. In addition, the US Department of Health and Human Services reports that 30–40% of Americans are infected with H. pylori infection. Further, socioeconomic factors, such as low income, lack of education, household crowding, and immigration contribute to H. pylori infection among Hispanic and Black populations in the US. These statistics determine an increase in the need for non-invasive diagnostics for H. pylori in the US, thus, boosting the growth of the US Helicobacter pylori non-invasive testing market.
Based on test type, the US Helicobacter pylori non-invasive testing market is categorized into urea breath, stool antigen, and serology. In 2022, the urea breath segment held the largest share of the US Helicobacter pylori non-invasive testing market. Moreover, the same segment is expected to grow at the fastest rate during the coming years. A urea breath test (UBT) is one of the essential noninvasive methods for detecting H. pylori infection. The test involves detecting H. pylori based on its ability to produce urease enzyme, which results in the hydrolysis of orally administered urea into carbon dioxide and ammonia. These gases diffuse into the blood, and the lungs then excrete them. UBTs are reliable, low-burden tests validated in adults and children. Thus, these contribute to the growth of US Helicobacter pylori non-invasive testing market size.
According to Cancer Research UK, chronic bacterial infection causes long-lasting stomach inflammation, also known as severe chronic atrophic gastritis or SCAG; prolonged stomach ulcers can even lead to cancer. According to the World Health Organization (WHO), cancer is a significant cause of death across the world; approximately 1.9 million new cancer cases were recorded in the world in 2021. Colorectal cancer is a common type, with 146,589 new cases recorded in 2022. This type of cancer is more prevalent in middle-aged and geriatric populations. It rarely occurs among people aged less than 40 with genetic predisposition or predisposing conditions. The early detection of H. pylori via appropriate tests is necessary to prevent colorectal cancer. Early diagnosis is pivotal to reducing cancer-related mortality rates. Thus, the rising cases of bacterial infections are necessitating urea breath tests which contributes to the growth of US Helicobacter Pylori non-invasive testing market.
In February 2020, Gulf Coast Scientific received approval from the US Food and Drug Administration (FDA) for the Pylo Plus UBT System, a 13C UBT for the detection of H. pylori infection. In addition, key players in the US Helicobacter pylori non-invasive testing market form alliances to introduce new products and expand their presence. For instance, in 2021, Meridian Bio signed an agreement to acquire BreahTrek from Otsuka Pharmaceutical for US$ 20 million. Such collaborations led to the new product launches in the UBT segment and contribute the overall growth of US Helicobacter pylori non-invasive testing market during the forecast period.
Strategic insights for the US Helicobacter Pylori Non-Invasive Testing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
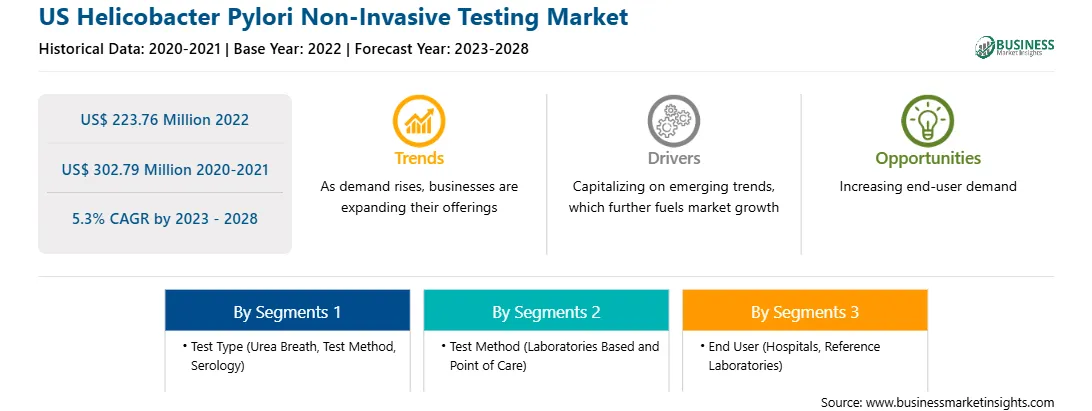
| Market size in 2022 | US$ 223.76 Million |
| Market Size by 2028 | US$ 302.79 Million |
| CAGR (2023 - 2028) | 5.3% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Test Type
|
| Regions and Countries Covered | United States
|
| Market leaders and key company profiles |
|
The geographic scope of the US Helicobacter Pylori Non-Invasive Testing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The US Helicobacter Pylori Non-Invasive Testing Market is valued at US$ 223.76 Million in 2022, it is projected to reach US$ 302.79 Million by 2028.
As per our report US Helicobacter Pylori Non-Invasive Testing Market, the market size is valued at US$ 223.76 Million in 2022, projecting it to reach US$ 302.79 Million by 2028. This translates to a CAGR of approximately 5.3% during the forecast period.
The US Helicobacter Pylori Non-Invasive Testing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the US Helicobacter Pylori Non-Invasive Testing Market report:
The US Helicobacter Pylori Non-Invasive Testing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The US Helicobacter Pylori Non-Invasive Testing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the US Helicobacter Pylori Non-Invasive Testing Market value chain can benefit from the information contained in a comprehensive market report.