Market Introduction
The North America Cervical Cancer Diagnostic Testing Market has been segmented into the US, Canada, and Mexico. The US held the largest share of the North America Cervical Cancer Diagnostic Testing Market in 2021. In North America, the US holds a significant share of the Cervical Cancer Diagnostic Testing market. The growth of the market in the country is primarily driven by a vast number of R&D activities; financial aids by governments, other private and non-private organizations; collaborations within pharmaceuticals and technology companies; and increasing prevalence of neurological diseases in the country. Additionally, increasing the active participation of the government organizations in enhancing the testing procedures is expected to boost the market during the forecast period. Furthermore, the implementation of regulatory policies for rare diseases is likely to offer a significant opportunity for the market's growth during the forecast period. For instance, the American Cancer Society (ACS) in September 2020 updated the guidelines for cervical cancer screening. Thus, such active involvement organization is expected to support the market growth. Moreover, as per the World Health Organization - Cervical Cancer Country Profiles, 2021, the age-standardized cervical cancer incidence rate per 100 000 women in 2020 is estimated to be 6.2. The US health system is witnessing a massive number of product innovations for the testing of chronic conditions and rare diseases, which would, in turn, drive the growth of the market in the US during the forecast period.
In case of COVID-19, North America is highly affected the US. The chaotic situation was created in the medical industry across the countries, increased demand for diagnosing and therapeutic devices have dramatically increased in the hospitals. For instance, the demand for ventilators, respirators, and in vitro diagnostic (IVD) tests has risen drastically in hospitals across the countries in the region. The FDA has increased its efforts to support the health of people and has imposed several guidelines and for hospitals and medical device companies. Various companies also enhanced their research and development activities for diagnostics tests and therapeutic devices. Due to the fear of infection of COVID19, women are hesitating to take cervical cancer testing such as the PAP test, biopsies, colonoscopy, and others, as it generally requires an actual visit to the healthcare. This trend among the patients is expected to affect the cervical cancer diagnostic testing market in the region. However, healthcare providers are now offering at-home testing services for the initial diagnosis through point-of-care devices and kits. The market players are actively involved in organic and inorganic developments. Thus, the outbreak of COVID-19 has shown a remarkable impact on market growth in the region.
Strategic insights for the North America Cervical Cancer Diagnostic Testing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the North America Cervical Cancer Diagnostic Testing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.North America Cervical Cancer Diagnostic Testing Strategic Insights
North America Cervical Cancer Diagnostic Testing Report Scope
Report Attribute
Details
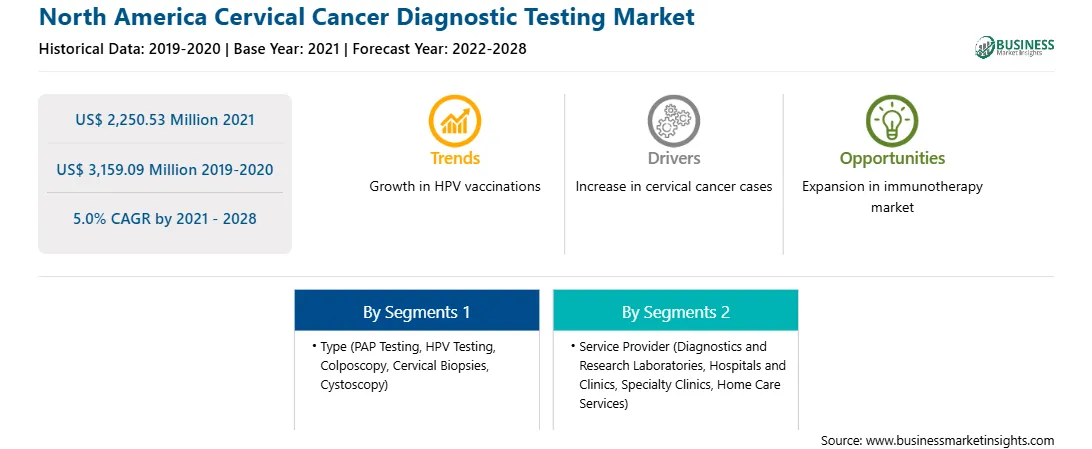
Market size in 2021
US$ 2,250.53 Million
Market Size by 2028
US$ 3,159.09 Million
CAGR (2021 - 2028) 5.0%
Historical Data
2019-2020
Forecast period
2022-2028
Segments Covered
By Type
By Service Provider
Regions and Countries Covered
North America
Market leaders and key company profiles
North America Cervical Cancer Diagnostic Testing Regional Insights
Market Overview and Dynamics
The North America cervical cancer diagnostic testing market is expected to grow from US$ 2,250.53 million in 2021 to US$ 3,159.09 million by 2028; it is estimated to grow at a CAGR of 5.0% from 2021 to 2028. Healthcare players concentrate on several countries, owing to the large population suffering from cancer in these countries. Moreover, with rising production costs against their practices, these companies strive to produce sufficient revenue to entertain their investors. Having presence in growing countries offers reliable and profitable growth opportunities to the major players to lower their operating costs and expand their geographic reach. Advancements in biotechnology have increased the demand for improved diagnostics based on analytical systems in the healthcare market in several countries; they are also easing the shift toward point of care diagnostics kits. Fields such as infectious disease testing, molecular oncology, and pharmacogenomics in several countries open new growth avenues. Hence, healthcare companies are targeting patients or consumers from several economies. Companies in the healthcare sector have been investing significant amounts of their revenue in R&D activities to develop better and advanced offerings and technologies. Several countries across the region are going at a good rate. Several activities in the region propel the demand for metabolomics-related services. Thus, the rising prevalence of cancer and technological advancements in several economies are boosting the cervical cancer diagnosis test market and providing significant growth opportunities to the players operating in the North America market.
Key Market Segments
In terms of type, the PAP testing segment accounted for the largest share of the North America cervical cancer diagnostic testing market in 2020. In terms of service provider, the diagnostics and research laboratories segment held a larger market share of the North America cervical cancer diagnostic testing market in 2020.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on the North America cervical cancer diagnostic testing market are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Abbott; BD; Cooper Companies, Inc.; DYSIS Medical Inc; F. Hoffmann-La Roche Ltd.; Femasys Inc.; Guided Therapeutics, Inc; Hologic, Inc.; QIAGEN; and Quest Diagnostics Incorporated among others.
Reasons to buy report
North America Cervical Cancer Diagnostic Testing Market Segmentation
North America Cervical Cancer Diagnostic Testing Market - By Type
North America Cervical Cancer Diagnostic Testing Market -
By Service Provider
North America Cervical Cancer Diagnostic Testing Market - By Country
North America Cervical Cancer Diagnostic Testing Market - Company Profiles
The North America Cervical Cancer Diagnostic Testing Market is valued at US$ 2,250.53 Million in 2021, it is projected to reach US$ 3,159.09 Million by 2028.
As per our report North America Cervical Cancer Diagnostic Testing Market, the market size is valued at US$ 2,250.53 Million in 2021, projecting it to reach US$ 3,159.09 Million by 2028. This translates to a CAGR of approximately 5.0% during the forecast period.
The North America Cervical Cancer Diagnostic Testing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Cervical Cancer Diagnostic Testing Market report:
The North America Cervical Cancer Diagnostic Testing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Cervical Cancer Diagnostic Testing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Cervical Cancer Diagnostic Testing Market value chain can benefit from the information contained in a comprehensive market report.