North America Cardiac Markers Market
No. of Pages: 106 | Report Code: BMIRE00030328 | Category: Life Sciences
No. of Pages: 106 | Report Code: BMIRE00030328 | Category: Life Sciences
The North America cardiac marker market forecast presented in this report can help stakeholders in this marketplace plan their growth strategies. The rising prevalence of cardiovascular diseases and a surge in the demand for point-of-care cardiac testing kits are the key factors propelling the North America cardiac marker market growth.
Hypoxia significantly affects exosome cargo and produces various proteins and miRNAs that indicate angiogenesis, growth, and progression for CVD through different signaling pathways. Exosomes has gathered the attention of researchers because they are relevant to intercellular communication under both physiological and pathological conditions. The upregulated exosome miRNAs miR-133a, miR-208a, miR-1, miR-499-5p, and miR-30a have been designated for the timely diagnosis of acute myocardial infarction (AMI). Further, derived exosomes namely, miR-192, miR-146a, miR-194, and miR-92b-5p are considered as potential biomarkers for HF.
Biomarker | Disease | Function |
miRNA-126, miRNA-223, and miRNA-320b | Acute myocardial infarction | Platelet activation and thrombus formation, endothelial damage, myocardial apoptosis, and fibroblast proliferation |
miRNA-1, miRNA-21a/b, and miRNA-29b | Acute myocardial infraction | Myocardial apoptosis, fibroblast proliferation, and cardiac hypertrophy |
miRNA-208a | Acute myocardial infarction | Cardiac hypertrophy and electrical conduction |
miRNA-499 | Acute myocardial infarction | Myocardial apoptosis |
miRNA-486 | Acute myocardial infarction | Myocardial apoptosis (protective) |
miRNA-223-5p | Acute myocardial infarction, atherosclerosis, and heart failure | Cell proliferation, migration, apoptosis, and polarization; cardiomyocyte hypertrophy; and electrical conduction |
miRNA-941 | Acute coronary syndrome | Cell proliferation and inflammation |
miRNA-216a and miRNA-451 | Coronary artery disease | Endothelial damage and monocyte recruitment |
miRNA-223-3p, miRNA-122-5p, and miRNA-93-5p | Coronary artery disease | Inflammation, migration and apoptosis, cardiomyocyte hypertrophy, electrical conduction, and cardiomyocyte apoptosis |
miRNA-142-3p, miRNA-17-5p, and miRNA-126 | Acute myocardial infarction and coronary artery disease | Inflammation; cardiomyocyte hypertrophy; and cell proliferation, migration, and apoptosis |
miRNA-133a | Coronary artery disease | Cell proliferation and differentiation, cardiac hypertrophy, and electrical conduction (arrhythmia) |
Serpin G1, Serpin F2, and Cystatin C CD14 | Heart failure and acute coronary syndrome | Inflammation, decrease in kidney function, decrease in fibrinolysis, and thrombotic process |
Source: Allen Press
In coronary artery disease (CAD) patients, upregulated exosome proteins—including fibrinogen beta/gamma chain, alpha-1-antichymotrypsin, and inter-alpha-trypsin inhibitor heavy chain—were evaluated as putative protein biomarkers. The function of exosomes in CVD pathogenesis through diverse intercellular communication mechanisms is gaining significant recognition. Several studies conducted in recent years have generated evidence supporting the association of exosomes with normal physiology (cardiac development, reticulocyte maturation, and myocardial angiogenesis) and pathophysiological processes, including ischemia/reperfusion (IR) injury, atherosclerosis, and cardiac remodeling. Stressful conditions such as hypoxia and inflammation can modulate biological exosome content and target cells, thereby contributing to improving or impairing cardiac function. The integration of exosome-based miRNAs and proteins in body fluids enables a comprehensive analysis of the potential biomarker role of these components in cardiovascular diseases. Therefore, the use of exosome biomarkers is emerging as a new approach for the diagnosis of CVDs.
Companies can submit an application for regulatory qualification for a cardiac marker to the FDA Biomarker Qualification Program for a specific application. Only the qualified marker can be used in multiple drug development programs without the need for the Center for Drug Evaluation and Research (CDER) approval to reconfirm the suitability of the markers.
Cardiac markers are widely used in epidemiological studies for completing the investigation of numerous stages of CVD. The process requires more careful handling and storage of valuable biological samples to obtain detailed information. Precise quality control measures are required to ensure that these samples are handled in appropriate storage conditions to avoid data loss. Cardiac marker studies depend on the integrity of samples and the manner of collection, processing, and storage, as archived samples are used in these studies. Handling, labeling, processing, aliquoting, storage, and transportation may affect study results. The sample must be placed on dry ice and shipped the same day. If the process is not carried out properly, it can impact the quality of the sample, subsequently hampering the outcomes. Therefore, strict validation protocols established by the US FDA and technical issues associated with sample collection and storage hamper the growth of the North America cardiac markers market.
The the developed healthcare system and the high acceptance of cardiac markers for diagnosing and predicting diseases. The growing geriatric population needs biomarker testing for detecting conditions such as acute myocardial infarction, thereby driving demand for testing products. The existence of key players such as Quidel Corporation and Danaher Corporation in the region is another factor contributing to the growth of this market. According to the World Health Organization (WHO), ~77 million adults over the age of 18 have type 2 diabetes, including 25 million prediabetes, which increases the possibility of cardiovascular diseases (CVDs) and puts additional pressure on the healthcare system. Furthermore, increasing strategies adopted by market players in this region to develop innovative cardiac biomarker tests are expected to contribute to the market growth in this region.
A few of the major primary and secondary sources referred to while preparing the report on the North America cardiac marker market are the World Bank Data, National Health Service (NHS), US Department of Health and Human Services (HHS), and WHO (World Health Organization).
Strategic insights for the North America Cardiac Markers provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 1.17 Billion |
| Market Size by 2031 | US$ 2.86 Billion |
| CAGR (2023 - 2031) | 11.8% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
|
The geographic scope of the North America Cardiac Markers refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The List of Companies - North America Cardiac Markers Market
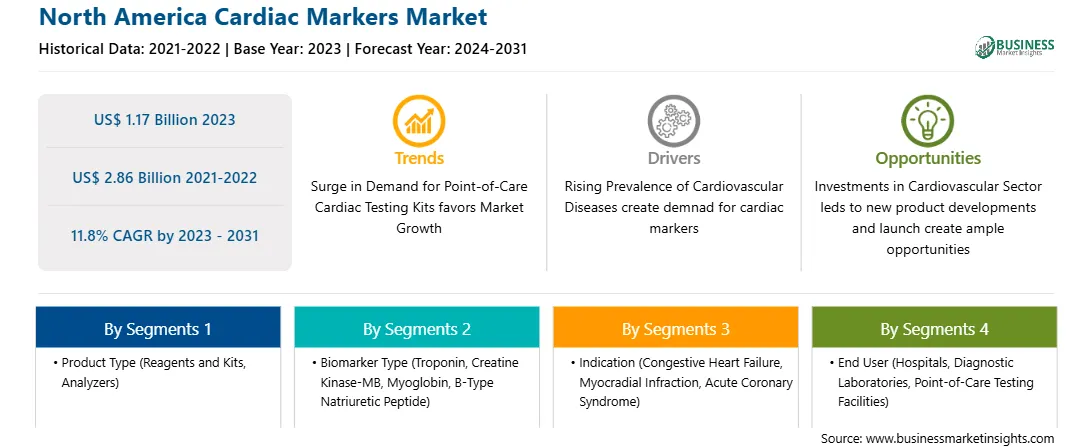
The North America Cardiac Markers Market is valued at US$ 1.17 Billion in 2023, it is projected to reach US$ 2.86 Billion by 2031.
As per our report North America Cardiac Markers Market, the market size is valued at US$ 1.17 Billion in 2023, projecting it to reach US$ 2.86 Billion by 2031. This translates to a CAGR of approximately 11.8% during the forecast period.
The North America Cardiac Markers Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Cardiac Markers Market report:
The North America Cardiac Markers Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Cardiac Markers Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Cardiac Markers Market value chain can benefit from the information contained in a comprehensive market report.