The Asia Pacific custom antibody services market size is expected to reach US$ 167.86 million by 2031 from US$ 68.24 million in 2023. The market is estimated to record a CAGR of 11.9% from 2023 to 2031.
Asia Pacific is the fastest-growing market for custom antibody services across the world. The Asia Pacific custom antibody services market is segmented into China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific. China, India, and Japan are three significant contributors to the market growth in this region, owing to the rise in geriatric population, increasing chronic and infectious disease prevalence, and burgeoning demand for advanced medical technologies. Additionally, the collaborative efforts of market players to offer more efficient custom antibody services, coupled with the rapidly growing pharmaceutical and biotechnology industries, support the growth of the custom antibody services market in Asia Pacific.
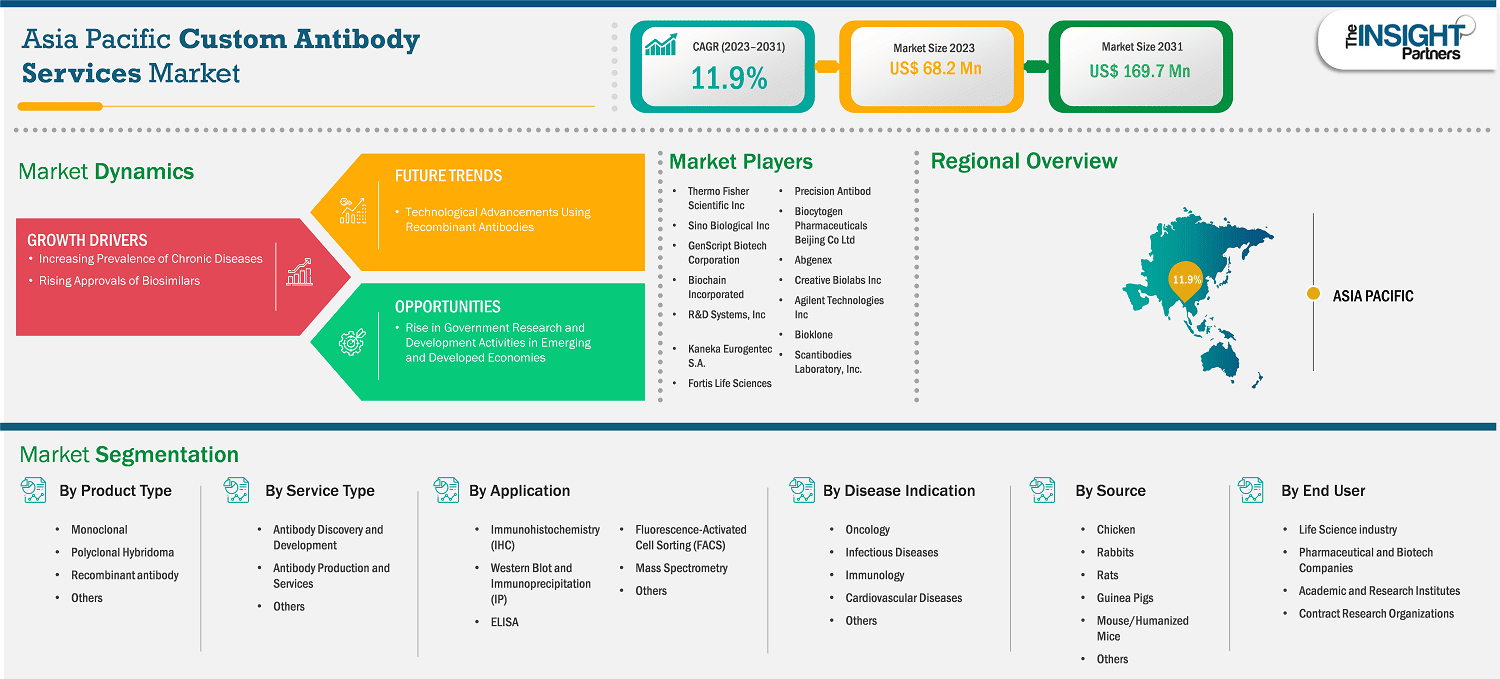
Key segments that contributed to the derivation of the Asia Pacific custom antibody services market analysis are product type, service type, application, disease indication, source, and end user.
The Food and Drug Administration (FDA) approves biosimilar products and provides the scientific and regulatory advice needed to introduce safe and effective biosimilars to the market. The approval of biosimilar products can improve patient care by increasing the number of medication options at potentially lower costs.
A few recent approvals of biosimilar products are mentioned below:
|
Biosimilars Name |
Approval Date |
Reference Product |
|
Alymsys (bevacizumab-maly) |
April 2022 |
Avastin (bevacizumab) |
|
Cimerli (ranibizumab-eqrn) |
August 2022 |
Lucentis (ranibizumab) |
|
Fylnetra (pegfilgrastim-pbbk) |
May 2022 |
Neulasta (pegfilgrastim) |
|
Stimufend (pegfilgrastim-fpgk) |
September 2022 |
Neulasta (pegfilgrastim) |
|
Vegzelma (bevacizumab-adcd) |
September 2022 |
Avastin (bevacizumab) |
|
Idacio (adalimumab-aacf) |
December 2022 |
Humira (adalimumab) |
|
Byooviz |
September 2021 |
Lucentis (ranibizumab) |
|
Rezvoglar |
December 2021 |
Lantus (insulin glargine) |
|
Semglee |
July 2021 |
Lantus (Insulin glargine) |
|
Yusimry (adalimumab-aqvh) |
December 2021 |
Humira (adalimumab) |
|
Hulio |
July 2020 |
Humira (adalimumab) |
|
Riabni |
December 2020 |
Rituxan (rituximab) |
|
Nyvepria |
June 2020 |
Neulasta (pegfilgrastim) |
Biosimilars can reduce treatment costs and improve patient access to biologic therapies. In recent years, a vast range of biosimilars have been approved by the FDA. According to ScienceDirect.com, biosimilars can retain costs and expand the availability of monoclonal antibodies. Thus, the rising approvals of biosimilars fuel the growth of the custom antibody services market.
Based on country, the Asia Pacific custom antibody services market comprises Japan, China, India, South Korea, Australia, and the Rest of Asia Pacific. China held the largest share in 2023.
China is home to various medical, pharmaceutical, and biotechnology companies operating across the world. It has over ~300 medical contract research organizations (CROs) along with prominent biopharmaceutical product manufacturers. CROs utilize custom antibody services to develop highly specific antibodies for research and drug development. Custom antibodies help CROs provide precise and effective solutions for customer's specific research needs. In 2020, Bio-Thera Solutions (a Chinese biotech company) received US$ 241 million in funding from investors, including the China Life Science Fund, to support the development of its monoclonal antibodies pipeline. These antibodies are widely utilized in various applications, including drug discovery, diagnostics, and therapeutics. In March 2024, Sino Biological, Inc., which provides biological research reagents and contract research services, formed a services partnership with Toronto-based Rapid Novor, Inc. Under the agreement terms, Sino Biological would market Rapid Novor's proprietary de novo REmAb monoclonal antibody sequencing service in combination with its custom monoclonal antibody development and production services. Thus, an upsurge in funding initiatives from governments and investors, and strategic efforts by companies hold potential growth opportunities for the custom antibody services market in China.
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 68.24 Million |
| Market Size by 2031 | US$ 167.86 Million |
| CAGR (2023 - 2031) | 11.9% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
Asia Pacific
|
| Market leaders and key company profiles |
|
Some of the key players operating in the market include Thermo Fisher Scientific Inc; Sino Biological Inc.; GenScript Biotech Corporation; Biochain Incorporated; R&D Systems, Inc; Kaneka Eurogentec S.A.; Fortis Life Sciences; Precision Antibody (A&G Pharmaceutical, Inc.; Biocytogen Pharmaceuticals Beijing Co Ltd; Abgenex; Creative Biolabs Inc; Agilent Technologies Inc; Bioklone; and Scantibodies Laboratory, Inc. among others. These players are adopting various strategies such as expansion, product innovation, and mergers and acquisitions to provide innovative products to their consumers and increase their market share.
The following methodology has been followed for the collection and analysis of data presented in this report:
The research process begins with comprehensive secondary research, utilizing both internal and external sources to gather qualitative and quantitative data for each market. Commonly referenced secondary research sources include, but are not limited to:
Note: All financial data included in the Company Profiles section has been standardized to USD. For companies reporting in other currencies, figures have been converted to USD using the relevant exchange rates for the corresponding year.
The Insight Partners conducts a significant number of primary interviews each year with industry stakeholders and experts to validate its data analysis and gain valuable insights. These research interviews are designed to:
Primary research is conducted via email interactions and telephone interviews, encompassing various markets, categories, segments, and sub-segments across different regions. Participants typically include:
The Asia Pacific Custom Antibody Services Market is valued at US$ 68.24 Million in 2023, it is projected to reach US$ 167.86 Million by 2031.
As per our report Asia Pacific Custom Antibody Services Market, the market size is valued at US$ 68.24 Million in 2023, projecting it to reach US$ 167.86 Million by 2031. This translates to a CAGR of approximately 11.9% during the forecast period.
The Asia Pacific Custom Antibody Services Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Custom Antibody Services Market report:
The Asia Pacific Custom Antibody Services Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Custom Antibody Services Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Custom Antibody Services Market value chain can benefit from the information contained in a comprehensive market report.
Please tell us your area of interest
(Market Segments/ Regions and Countries/ Companies)