Middle East and Africa Healthcare Regulatory Affairs Outsourcing Market Forecast to 2028 - COVID-19 Impact and Regional Analysis By Service Type (Regulatory & Scientific Strategy Development, Medical & Scientific Writing, eCTD & e-Submissions, Data Management Services, Life Cycle Management Services, Pharmacovigilance, Chemistry Manufacturing & Controls (CMC) Services, Regulatory Labelling, and Regulatory Artwork Services) and End User (Pharmaceutical Companies, Biotechnology Companies, and Medical Devices Companies)
Market Introduction
Middle East & Africa consists of three major countries namely United Arab Emirates (UAE), Saudi Arabia and South Africa. Market growth is expected due to increasing R&D expenditure. Thus, depreciation pressures are fueling the regulatory outsourcing trend. In addition, contract analysis organizations provide price-efficient solutions and compliance with health authority requirements, reimbursement scenario changes, and pricing pressures and developments from market participants in medical outsourcing technology in countries like Saudi Arabia, the United Arab Emirates, and South Africa. Countries are developing their health facilities, investing heavily in novel drug therapy research, and increasing partnerships with foreign companies are likely to fuel market growth over the forecast period. Soaring market consolidation activities is the major factor driving the growth of the MEA healthcare regulatory affairs outsourcing market.
COVID-19 has had severe effect on South Africa, Israel, the UAE, and Saudi Arabia, among other countries in the Middle East and Africa. The pandemic has increased demand for home-care medical devices. The region is witnessing an increase in the number of patients being admitted to intensive care units (ICU), an increasing number of drugs that pose multiple diagnostic and therapeutic challenges to strained health systems, leading to an increase in medical devices. The Middle East and Africa, like the other regions, also experienced three waves of the pandemic. With the pandemic not over yet, governments must take steps to prevent it from spreading, protect hospitals from traffic jams, and ensure uncompromising care for all patients. The countries in the MEA region have taken strict precautionary measures to control the spread of COVID-19. Countries such as the UAE, Saudi Arabia, Jordan, Iraq, and Iran have strictly imposed lockdowns. The pharmaceutical market experiences different dynamics in different MENA countries. For example, high purchasing power and a cultural predilection for expensive foreign brands in Saudi Arabia compel them to import a large percentages (85%) of pharmaceutical products. The inclination of South Africa toward generics has increased in the recent years. Additionally, various healthcare companies are likely to refer to more regulatory advice during the recovery phase, post-pandemic, especially focusing on remote monitoring, telemedicine, data protection, etc. Also, with the growing emphasis on accelerating product development and obtaining quick approvals for therapies, vaccines, and devices effective against COVID-19, companies are likely to work even more closely with regulators. Medical matters play a central role in overcoming barriers to accessing health care professionals (HCPs). In addition, due to the compliance requirements, Medical Affairs is responsible for providing the HCPs in the MEA with unbiased and transparent medical information in real-time.
Get more information on this report :
Market Overview and Dynamics
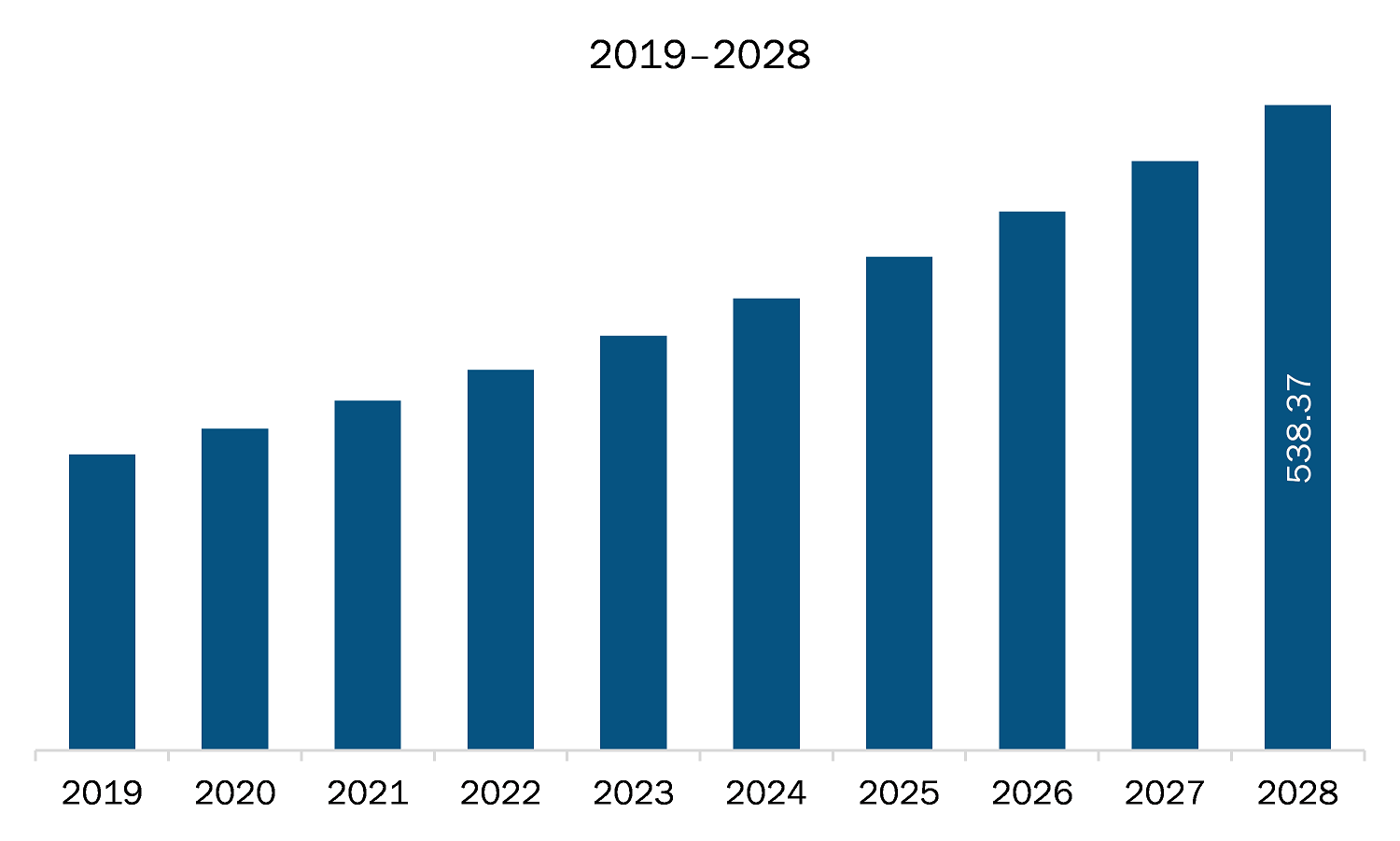
The healthcare regulatory affairs outsourcing market in MEA is expected to grow from US$ 291.72 million in 2021 to US$ 538.37 million by 2028; it is estimated to grow at a CAGR of 9.1% from 2021 to 2028. Healthcare regulatory affairs outsourcing functions have become challenging across the world. The increasing demand to obtain approval for new products, focusing more on core strengths with minimum operational cost and maintain compliance have increased during the last decade. Healthcare regulatory approval processes have become more stringent, and time consuming and market players are striving to obtain approval for their product in the first attempt to acquire greater market shares. Due to strictness by regulatory agencies in developed countries and constantly changing regulatory landscape in developing countries, companies are compelled to start in house regulatory department, or they opt to outsource the regulatory activities. It is not feasible option to establish in house regulatory affairs department to most of the healthcare companies as it would increase the operational costs and decrease the revenue, therefore companies are increasingly adopting outsourcing model based on the size and priority of the projects. Subsequently, the investments in the regulatory information systems have increased significantly to keep pace with the need to automate activities like regulatory publishing and operations. In such scenario, outsourcing has steadily become an important part of the healthcare regulatory affairs, which is expected to raise demand for healthcare regulatory affairs outsourcing, thereby driving the market growth.
Key Market Segments
The MEA healthcare regulatory affairs outsourcing market has been segmented based on service type, end user, and country. On the basis of service type, the MEA healthcare regulatory affairs outsourcing market is segmented into medical & scientific writing, pharmacovigilance, data management services, life cycle management services, eCTD and e-Submissions, regulatory and scientific strategy development, chemistry manufacturing and controls (CMC) services, regulatory labelling, and regulatory artwork services. The medical & scientific writing segment dominated the market in 2020 and pharmacovigilance segment is expected to be the fastest growing during the forecast period. Based on end user, the market is segmented into pharmaceutical companies, biotechnology companies, and medical devices companies. The pharmaceutical companies segment dominated the market in 2020 and is expected to be the fastest growing during the forecast period. Likewise, the medical devices companies segmented is categorized into medical device materials & biomaterials, medical device, biomarkers and in vitro diagnostics (IVD), medical device software (SaMD), medical device electromechanics, medical device substance-based, and medical device of combination product.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on healthcare regulatory affairs outsourcing market in MEA are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Arriello Ireland Ltd., IQVIA Inc., PAREXEL INTERNATIONAL CORPORATION, PHARMALEX GMBH, and ProductLife Group are among others.
Reasons to buy report
- To understand the MEA healthcare regulatory affairs outsourcing market landscape and identify market segments that are most likely to guarantee a strong return
- Stay ahead of the race by comprehending the ever-changing competitive landscape for MEA healthcare regulatory affairs outsourcing market
- Efficiently plan M&A and partnership deals in MEA healthcare regulatory affairs outsourcing market by identifying market segments with the most promising probable sales
- Helps to take knowledgeable business decisions from perceptive and comprehensive analysis of market performance of various segment form MEA healthcare regulatory affairs outsourcing market
- Obtain market revenue forecast for market by various segments from 2021-2028 in MEA region.
MEA Healthcare Regulatory Affairs Outsourcing Market Segmentation
MEA Healthcare Regulatory Affairs Outsourcing Market –By Service Type
- Medical & Scientific Writing
- Pharmacovigilance
- Data Management Services
- Life Cycle Management Services
- eCTD and e-Submissions
- Regulatory and Scientific Strategy development
- Chemistry Manufacturing and Controls (CMC) Services
- Regulatory Labelling
- Regulatory Artwork Services
MEA Healthcare Regulatory Affairs Outsourcing Market –By End User
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Devices Companies
- Medical Device Materials & Biomaterials
- Medical Device Biomarkers and In vitro Diagnostics (IVD)
- Medical Device Software (SaMD)
- Medical Device Electromechanics
- Medical Device Substance-based
- Medical Device of Combination Product
MEA Healthcare Regulatory Affairs Outsourcing Market -By Country
- Saudi Arabia
- South Africa
- UAE
- Rest of MEA
MEA Healthcare Regulatory Affairs Outsourcing Market -Company Profiles
- Arriello Ireland Ltd.
- IQVIA Inc.
- PAREXEL INTERNATIONAL CORPORATION
- PHARMALEX GMBH
- ProductLife Group
TABLE OF CONTENTS
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.3 Market Segmentation
1.3.1 MEA Healthcare Regulatory Affairs Outsourcing Market – By Service Type
1.3.2 MEA Healthcare Regulatory Affairs Outsourcing Market – By End User
1.3.3 MEA Healthcare Regulatory Affairs Outsourcing Market – By Country
2. MEA Healthcare Regulatory Affairs Outsourcing Market – Key Takeaways
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. MEA Healthcare Regulatory Affairs Outsourcing Market – Market Landscape
4.1 Overview
4.1.1 Middle East And Africa– PEST Analysis
4.2 Expert Opinions
5. MEA Healthcare Regulatory Affairs Outsourcing Market– Key Market Dynamics
5.1 Market Drivers
5.1.1 Growing Regulatory Pressure on Healthcare Companies
5.1.2 Intensified Demand for Speedy Approval of New Products
5.2 Market Restraints
5.2.1 Dearth of Skilled Professionals
5.3 Market Opportunities
5.3.1 Progress in Specialty Therapies, Orphan Drugs, and Personalized Medicines
5.4 Future Trends
5.4.1 Soaring Market Consolidation Activities
5.5 Impact Analysis
6. Healthcare Regulatory Affairs Outsourcing Market – MEA Analysis
6.1 MEA Healthcare Regulatory Affairs Outsourcing Market Revenue Forecast and Analysis
7. MEA Healthcare Regulatory Affairs Outsourcing Market Analysis – By Service Type
7.1 Overview
7.2 Healthcare Regulatory Affairs Outsourcing Market, by Service Type (2021 and 2028)
7.3 Regulatory and Scientific Strategy Development
7.3.1 Overview
7.3.2 Regulatory and Scientific Strategy Development: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.4 Medical and Scientific Writing
7.4.1 Overview
7.4.2 Medical and Scientific Writing: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.5 eCTD and E-Submissions
7.5.1 Overview
7.5.2 eCTD and E-Submissions: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.6 Data Management Services
7.6.1 Overview
7.6.2 Data Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.7 Life Cycle Management Services
7.7.1 Overview
7.7.2 Life Cycle Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.8 Pharmacovigilance
7.8.1 Overview
7.8.2 Pharmacovigilance : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.9 Chemistry Manufacturing and Controls (CMC) Services
7.9.1 Overview
7.9.2 Chemistry Manufacturing & Controls ((CMC) Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.10 Regulatory Labelling
7.10.1 Overview
7.10.2 Regulatory Labelling : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.11 Regulatory Artwork Services
7.11.1 Overview
7.11.2 Regulatory Artwork Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8. MEA Healthcare Regulatory Affairs Outsourcing Market Analysis – By End User
8.1 Overview
8.2 Healthcare Regulatory Affairs Outsourcing Market, by End-User (2020 and 2028)
8.3 Pharmaceutical Companies
8.3.1 Overview
8.3.2 Pharmaceutical Companies: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8.4 Biotechnology Companies
8.4.1 Overview
8.4.2 Biotechnology Companies : Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8.5 Medical Device Companies
8.5.1 Overview
8.5.2 Medical Device Companies: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8.5.2.1 Medical Device Software (SaMD) Market
8.5.2.1.1 Overview
8.5.2.1.2 Medical Device Software (SaMD) Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.2 Medical Device Materials & Biomaterials Market
8.5.2.2.1 Overview
8.5.2.2.2 Medical Device Materials & Biomaterials Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.3 Medical Device Biomarkers and In-vitro Diagnostics (IVD) Market
8.5.2.3.1 Overview
8.5.2.3.2 Medical Device Biomarkers and In-vitro Diagnostics (IVD) Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.4 Medical Device Electro mechanics Market
8.5.2.4.1 Overview
8.5.2.4.2 Medical Device Electro mechanics Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.5 Medical Device Substance Based Market
8.5.2.5.1 Overview
8.5.2.5.2 Medical Device Substance Based Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.6 Medical Device of Combination Product (DDC) Market
8.5.2.6.1 Overview
8.5.2.6.2 Medical Device of Combination Product (DDC) Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
9. MEA Healthcare Regulatory Affairs Outsourcing Market – Country Analysis
9.1 Overview
9.1.1 Middle East & Africa: Healthcare Regulatory Affairs Outsourcing Market, by Country, 2021 & 2028 (%)
9.1.1.1 Saudi Arabia: Healthcare Regulatory Affairs Outsourcing Market - Revenue and Forecast to 2028 (USD Million)
9.1.1.1.1 Saudi Arabia: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.1.2 Saudi Arabia Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
9.1.1.1.3 Saudi Arabia: Healthcare Regulatory Affairs Outsourcing Market, by End User – 2019–2028 (USD Million)
9.1.1.1.3.1 Saudi Arabia: Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies, 2019–2028 (USD Million)
9.1.1.2 UAE: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.2.1 UAE: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.2.2 UAE Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
9.1.1.2.3 UAE: Healthcare Regulatory Affairs Outsourcing Market, by End User – 2019–2028 (USD Million)
9.1.1.2.3.1 UAE: Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies, 2019–2028 (USD Million)
9.1.1.3 South Africa: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.3.1 South Africa: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.3.2 South Africa Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
9.1.1.3.3 South Africa: Healthcare Regulatory Affairs Outsourcing Market, by End User – 2019–2028 (USD Million)
9.1.1.3.3.1 South Africa: Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies, 2019–2028 (USD Million)
9.1.1.4 Rest of Middle East and Africa: Healthcare Regulatory Affairs Outsourcing Market - Revenue and Forecast to 2028 (USD Million)
9.1.1.4.1 Rest of Middle East and Africa: Healthcare Regulatory Affairs Outsourcing Market - Revenue and Forecast to 2028 (USD Million)
9.1.1.4.2 Rest of Middle East & Africa Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
9.1.1.4.3 Rest of Middle East & Africa: Healthcare Regulatory Affairs Outsourcing Market, by End User – 2019–2028 (USD Million)
9.1.1.4.3.1 Rest of Middle East & Africa: Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies, 2019–2028 (USD Million)
10. Impact Of COVID-19 Pandemic on MEA Healthcare Regulatory Affairs Outsourcing Market
11. Industry Landscape
11.1 Overview
11.2 Organic Developments
11.2.1 Overview
11.3 Inorganic Developments
11.3.1 Overview
12. Company Profiles
12.1 Arriello Ireland Ltd.
12.1.1 Key Facts
12.1.2 Business Description
12.1.3 Products and Services
12.1.4 Financial Overview
12.1.5 SWOT Analysis
12.1.6 Key Developments
12.2 PAREXEL INTERNATIONAL CORPORATION
12.2.1 Key Facts
12.2.2 Business Description
12.2.3 Products and Services
12.2.4 Financial Overview
12.2.5 SWOT Analysis
12.2.6 Key Developments
12.3 IQVIA Inc.
12.3.1 Key Facts
12.3.2 Business Description
12.3.3 Products and Services
12.3.4 Financial Overview
12.3.5 SWOT Analysis
12.3.6 Key Developments
12.4 PHARMALEX GMBH
12.4.1 Key Facts
12.4.2 Business Description
12.4.3 Products and Services
12.4.4 Financial Overview
12.4.5 SWOT Analysis
12.4.6 Key Developments
12.5 ProductLife Group
12.5.1 Key Facts
12.5.2 Business Description
12.5.3 Products and Services
12.5.4 Financial Overview
12.5.5 SWOT Analysis
12.5.6 Key Developments
13. Appendix
13.1 About The Insight Partners
13.2 Glossary of Terms
LIST OF TABLES
Table 1. MEA Healthcare Regulatory Affairs Outsourcing Market Revenue and Forecast to 2028 (US$ Million)
Table 2. Saudi Arabia Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
Table 3. Saudi Arabia Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
Table 4. Saudi Arabia Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies – Revenue and Forecast to 2028 (USD Million)
Table 5. UAE Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
Table 6. UAE Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
Table 7. UAE Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies – Revenue and Forecast to 2028 (USD Million)
Table 8. South Africa Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
Table 9. South Africa Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
Table 10. South Africa Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies – Revenue and Forecast to 2028 (USD Million)
Table 11. Rest of Middle East & Africa Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
Table 12. Rest of Middle East & Africa Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
Table 13. Rest of Middle East & Africa Healthcare Regulatory Affairs Outsourcing Market, by Medical Devices Companies – Revenue and Forecast to 2028 (USD Million)
Table 14. Organic Developments in the Healthcare Regulatory Affairs Outsourcing Market
Table 15. Inorganic Developments in the Healthcare Regulatory Affairs Outsourcing Market
Table 16. Glossary of Terms, MEA Healthcare Regulatory Affairs Outsourcing Market
LIST OF FIGURES
Figure 1. MEA Healthcare Regulatory Affairs Outsourcing Market Segmentation
Figure 2. MEA Healthcare Regulatory Affairs Outsourcing Market Segmentation, By Country
Figure 3. MEA Healthcare Regulatory Affairs Outsourcing Market Overview
Figure 4. Medical & Scientific Writing Segment Held the Largest Share of the Service Type Segment in MEA Healthcare Regulatory Affairs Outsourcing Market
Figure 5. Saudi Arabia to Show Significant Growth During Forecast Period
Figure 6. Middle East and Africa PEST Analysis
Figure 7. MEA Healthcare Regulatory Affairs Outsourcing Market Impact Analysis of Driver and Restraints
Figure 8. MEA Healthcare Regulatory Affairs Outsourcing Market – Revenue Forecast and Analysis – 2019- 2028
Figure 9. MEA Healthcare Regulatory Affairs Outsourcing Market Revenue Share, by Service Type (2020 and 2028)
Figure 10. MEA Regulatory and Scientific Strategy Development: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 11. MEA Medical and Scientific Writing: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 12. MEA eCTD and E-Submissions: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 13. MEA Data Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 14. MEA Life Cycle Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 15. MEA Pharmacovigilance : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 16. MEA Chemistry Manufacturing & Controls ((CMC) Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 17. MEA Regulatory Labelling: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 18. MEA Regulatory Artwork Services : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 19. MEA Healthcare Regulatory Affairs Outsourcing Market Revenue Share, by End-User (2020 and 2028)
Figure 20. MEA Pharmaceutical Companies : Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 21. MEA Biotechnology Companies: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 22. MEA Medical Device Companies: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 23. MEA Medical Device Software (SaMD) Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 24. MEA Medical Device Materials & Biomaterials Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 25. MEA Medical Device Biomarkers and In-vitro Diagnostics (IVD) Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 26. MEA Medical Device Electro mechanics Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 27. MEA Medical Device Substance Based Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 28. MEA Medical Device of Combination Product (DDC) Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 29. Middle East & Africa: Healthcare Regulatory Affairs Outsourcing Market, by Key Country – Revenue (2021) (USD Million)
Figure 30. Middle East & Africa: Healthcare Regulatory Affairs Outsourcing Market, by Country, 2021 & 2028 (%)
Figure 31. Saudi Arabia: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
Figure 32. UAE: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
Figure 33. South Africa: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
Figure 34. Rest of Middle East and Africa: Healthcare Regulatory Affairs Outsourcing Market - Revenue and Forecast to 2028 (USD Million)
Figure 35. Impact of COVID-19 Pandemic in Middle East and Africa Country Markets- Arriello Ireland Ltd.
- IQVIA Inc.
- PAREXEL INTERNATIONAL CORPORATION
- PHARMALEX GMBH
- ProductLife Group
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players and segments in the MEA Healthcare Regulatory Affairs Outsourcing market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the MEA Healthcare Regulatory Affairs Outsourcing market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth MEA market trends and outlook coupled with the factors driving the Healthcare Regulatory Affairs Outsourcing market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing and distribution