Asia Pacific Pharmacovigilance and Drug Safety Software Market Forecast to 2027 - COVID-19 Impact and Regional Analysis By Software Type (Adverse Event Reporting Software, Drug Safety Audits Software, Issue Tracking Software, and Fully Integrated Software); Delivery Mode (On-premise, and Cloud-based); End User (Pharmaceutical and Biotech Companies, Contract Research Organizations (CROs), and Business Process Outsourcing (BPO) Firms)
Market Introduction
Pharmacovigilance (PV) plays an essential role in the healthcare system through assessment, monitoring, and finding of drug interactions and their effects in human. Pharmacovigilance helps companies to monitor any adverse drug reaction events during the trial phase and also during the post marketing period.
Thus, the globalization of pharmacovigilance are expected to create a significant demand for pharmacovigilance and drug safety software in the coming years, which is further anticipated to drive the pharmacovigilance and drug safety software market.
Countries in Asia-Pacific are expecting to witness huge challenge due to growing COVID-19. Considering the economic operations and geographic condition, the outbreak of disease has affected on medical tourism, manufacturer of medical equipment, laser systems, accessories and other problems posed by shortage of healthcare infrastructure in Asia-Pacific low-income countries. After the first case in December in Wuhan, China, the coronavirus has spread to at least 180 countries and regions. To prevent the spread of disease, restrictive measures have been taken in countries such as India, South Korea, Singapore, Malaysia, and by the Philippines. According to WHO, due to the rapidly changing risk of COVID-19 affected countries and constantly controlling outbreak trends, any additional health measures are likely to significantly interfere with international travel and trade. There is vast variation in the reporting standards across the Asia Pacific. While some countries have adopted electronic reporting, many organizations still use conventional methods of courier or hand delivery for paper reporting or electronic reporting via compact disc. Currently, electronic reporting and email submissions are compatible with COVID-19. However, all methods require efficient communication between pharmacovigilance teams, local legal representative, courier support and teams dispersed across various geographical locations, for example portal entry in the local language.
Get more information on this report :
Market Overview and Dynamics
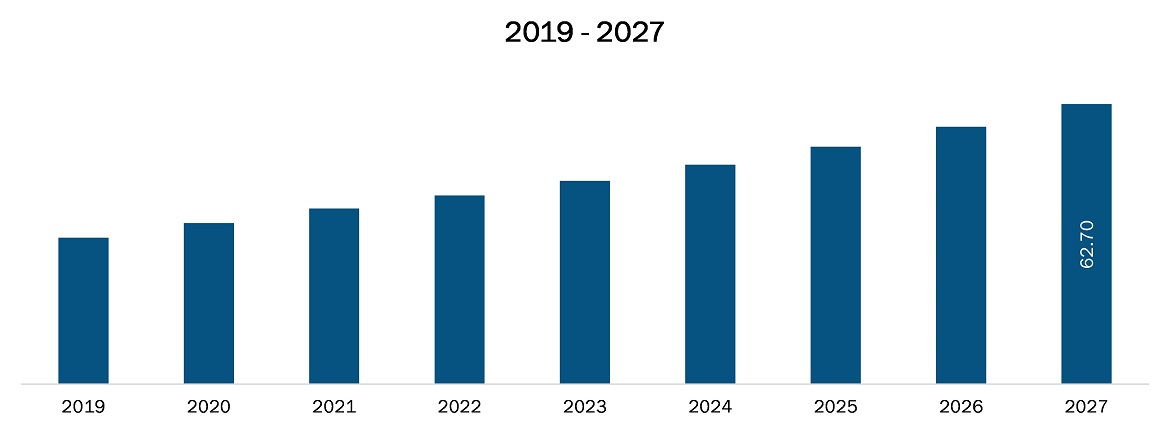
The pharmacovigilance and drug safety software market in Asia Pacific is expected to grow from US$ 32.8 million in 2019 to US$ 62.7 million by 2027; it is estimated to grow at a CAGR of 8.2% from 2020 to 2027. Adverse drug reactions (ADRs) is an important public health problem, signifying a significant cause of illness and death. Because all drugs have the potential for adverse drug reactions, a risk-benefit analysis is necessary whenever a drug is prescribed. ADR reported by a patient or healthcare professional adds to safety monitoring and thus to the safe and effective use of medicines. The increasing amount of data generated through adverse drug reaction report need to be handled and stored carefully. All these data come in different forms, language, location, etc. To arrange these uniformly the automation systems/software are helpful. Thus, the rising incidences of adverse drug reactions (ADRs) are likely to drive the market’s growth.
Key Market Segments
In terms of software type, the adverse event reporting software segment accounted for the largest share of the Asia Pacific pharmacovigilance and drug safety software market in 2019. In terms of delivery mode, the on-premise segment held a larger market share of the pharmacovigilance and drug safety software market in 2019. In terms of end user, the contract research organizations segment held a larger market share of the pharmacovigilance and drug safety software market in 2019.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on the Pharmacovigilance and drug safety software market in Asia Pacific are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Veeva Systems, IQVIA Inc., AB Cube, ArisGlobal LLC, Sparta Systems, Oracle Corporation, Sarjen Systems Pvt. Ltd, EXTEDO, and Online Business Applications, Inc.
Reasons to buy report
- To understand the Asia Pacific pharmacovigilance and drug safety software market landscape and identify market segments that are most likely to guarantee a strong return
- Stay ahead of the race by comprehending the ever-changing competitive landscape for Asia Pacific pharmacovigilance and drug safety software market
- Efficiently plan M&A and partnership deals in Asia Pacific pharmacovigilance and drug safety software market by identifying market segments with the most promising probable sales
- Helps to take knowledgeable business decisions from perceptive and comprehensive analysis of market performance of various segment form Asia Pacific pharmacovigilance and drug safety software market
- Obtain market revenue forecast for market by various segments from 2021-2028 in Asia Pacific region.
ASIA PACIFIC PHARMACOVIGILANCE AND DRUG SAFETY SOFTWARE MARKET SEGMENTATION
By Software Type
- Fully Integrated Software
- Adverse Event Reporting Software
- Drug Safety Audits Software
- Issue Tracking Software
By Delivery Mode
- Cloud-based
- On-premise
By End User
- Contract Research Organizations
- Pharmaceutical and Biotech Companies
- Business Process Outsourcing
By Country
- Asia Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia Pacific
Companies Mentioned
- Veeva Systems
- IQVIA Inc.
- AB Cube
- ArisGlobal LLC
- Sparta Systems
- Oracle Corporation
- Sarjen Systems Pvt. Ltd
- EXTEDO
- Online Business Applications, Inc.
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.3 Market Segmentation
1.3.1 Pharmacovigilance and Drug Safety Software Market – By Software Type
1.3.2 Pharmacovigilance and Drug Safety Software Market – By Delivery Mode
1.3.3 Pharmacovigilance and Drug Safety Software Market – By End User
1.3.4 Asia Pacific Pharmacovigilance and Drug Safety Software Market – By Country
2. Asia Pacific Pharmacovigilance and Drug Safety Software Market – Key Takeaways
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. Asia Pacific Pharmacovigilance and Drug Safety Software Market – Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 Pharmacovigilance and Drug Safety Software Market- Asia Pacific PEST Analysis
4.3 Expert Opinion
5. Asia Pacific Pharmacovigilance and Drug Safety Software Market - Key Market Dynamics
5.1 Market Drivers
5.1.1 Rising Incidences of Adverse Drug Reactions (ADRs)
5.1.2 Globalization of Pharmacovigilance
5.2 Market Restraints
5.2.1 Expensive Technology
5.3 Market Opportunities
5.3.1 Growth Opportunities in Developing Countries
5.4 Future Trends
5.4.1 Artificial Intelligence in Pharmacovigilance
5.5 Impact Analysis
6. Pharmacovigilance and Drug Safety Software Market – Asia Pacific Analysis
6.1 Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue Forecasts and Analysis
7. Asia Pacific Pharmacovigilance and Drug Safety Software Market Analysis and Forecasts To 2027 – By Software Type
7.1 Overview
7.2 Pharmacovigilance (PV) and Drug Safety Software Market, by Software Type, 2019 & 2027 (%)
7.3 Adverse Event Reporting Software Market
7.3.1 Overview
7.3.2 Adverse Event Reporting Software Market Revenue and Forecasts to 2027 (US$ Mn)
7.4 Drug Safety Audits Software
7.4.1 Overview
7.4.2 Drug Safety Audits Software Market Revenue and Forecasts to 2027 (US$ Mn)
7.5 Issue Tracking Software
7.5.1 Overview
7.5.2 Issue Tracking Software Market Revenue and Forecasts to 2027 (US$ Mn)
7.6 Fully Integrated Software
7.6.1 Overview
7.6.2 Fully Integrated Software Market Revenue and Forecasts to 2027 (US$ Mn)
8. Asia Pacific Pharmacovigilance and Drug Safety Software Market Analysis and Forecasts To 2027 – By Delivery Mode
8.1 Overview
8.2 Pharmacovigilance (PV) and Drug Safety Software Market, By Delivery Mode 2019 & 2027 (%)
8.3 On-premise
8.3.1 Overview
8.3.2 On-premise Market Revenue and Forecasts to 2027 (US$ Mn)
8.4 Cloud- Based
8.4.1 Overview
8.4.2 Cloud Based Market Revenue and Forecasts to 2027 (US$ Mn)
9. Asia Pacific Pharmacovigilance and Drug Safety Software Market Analysis and Forecasts to 2027– By End User
9.1 Overview
9.2 Pharmacovigilance (PV) and Drug Safety Software Market, By End User, 2019 & 2027 (%)
9.3 Pharmaceutical and Biotech Companies Market
9.3.1 Overview
9.3.2 Pharmaceutical and Biotech Companies Market Revenue and Forecasts to 2027 (US$ Mn)
9.4 Contract Research Organizations (CROs) Market
9.4.1 Overview
9.4.2 Contract Research Organizations (CROs) Market Revenue and Forecasts to 2027 (US$ Mn)
9.5 Business Process Outsourcing (BPO) Firms Market
9.5.1 Overview
9.5.2 Business Process Outsourcing (BPO) Firms Market Revenue and Forecasts to 2027 (US$ Mn)
10. Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 – Geographical Analysis
10.1 Asia Pacific: Pharmacovigilance and Drug Safety Software Market
10.1.1 Overview
10.1.2 Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Country (%)
10.1.3 Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027(US$ Mn)
10.1.3.1 Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.3.2 Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type (US$ Mn)
10.1.3.3 Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
10.1.3.4 Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
10.1.4 China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.4.1 China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.4.2 China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type (US$ Mn)
10.1.4.3 China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
10.1.4.4 China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
10.1.5 India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.5.1 India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.5.2 India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type (US$ Mn)
10.1.5.3 India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
10.1.5.4 India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
10.1.6 South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.6.1 South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.6.2 South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type (US$ Mn)
10.1.6.3 South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
10.1.6.4 South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
10.1.7 Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.7.1 Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.7.2 Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type (US$ Mn)
10.1.7.3 Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
10.1.7.4 Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
10.1.8 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.8.1 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
10.1.8.2 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type (US$ Mn)
10.1.8.3 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
10.1.8.4 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
11. Impact Of COVID-19 Pandemic on Asia Pacific Pharmacovigilance and Drug Safety Software Market
11.1 Asia Pacific: Impact Assessment of COVID-19 Pandemic
12. Industry Landscape
12.1 Overview
12.2 Growth Strategies in the Pharmacovigilance and Drug Safety Software Market
12.3 Organic Developments
12.3.1 Overview
12.3.2 Organic Developments Done by Companies
12.4 Inorganic Developments
12.4.1 Overview
12.4.2 Inorganic Growth Strategies by Companies
13. COMPANY PROFILES
13.1 Veeva Systems
13.1.1 Key Facts
13.1.2 Business Description
13.1.3 Products and Services
13.1.4 Financial Overview
13.1.5 SWOT Analysis
13.1.6 Key Developments
13.2 IQVIA Inc.
13.2.1 Key Facts
13.2.2 Business Description
13.2.3 Products and Services
13.2.4 Financial Overview
13.2.5 SWOT Analysis
13.2.6 Key Developments
13.3 AB Cube
13.3.1 Key Facts
13.3.2 Business Description
13.3.3 Products and Services
13.3.4 Financial Overview
13.3.5 SWOT Analysis
13.3.6 Key Developments
13.4 ArisGlobal LLC
13.4.1 Key Facts
13.4.2 Business Description
13.4.3 Products and Services
13.4.4 Financial Overview
13.4.5 SWOT Analysis
13.4.6 Key Developments
13.5 Sparta Systems
13.5.1 Key Facts
13.5.2 Business Description
13.5.3 Products and Services
13.5.4 Financial Overview
13.5.5 SWOT Analysis
13.5.6 Key Developments
13.6 Oracle Corporation
13.6.1 Key Facts
13.6.2 Business Description
13.6.3 Products and Services
13.6.4 Financial Overview
13.6.5 SWOT Analysis
13.6.6 Key Developments
13.7 Sarjen Systems Pvt. Ltd
13.7.1 Key Facts
13.7.2 Business Description
13.7.3 Products and Services
13.7.4 Financial Overview
13.7.5 SWOT Analysis
13.7.6 Key Developments
13.8 EXTEDO
13.8.1 Key Facts
13.8.2 Business Description
13.8.3 Products and Services
13.8.4 Financial Overview
13.8.5 SWOT Analysis
13.8.6 Key Developments
13.9 Online Business Applications, Inc.
13.9.1 Key Facts
13.9.2 Business Description
13.9.3 Products and Services
13.9.4 Financial Overview
13.9.5 SWOT Analysis
13.9.6 Key Developments
14. Appendix
14.1 About the Insight Partners
14.2 Glossary of Terms
LIST OF TABLES
Table 1. Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type(US$ Mn)
Table 2. Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
Table 3. Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
Table 4. China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type(US$ Mn)
Table 5. China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
Table 6. China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
Table 7. India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type(US$ Mn)
Table 8. India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
Table 9. India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
Table 10. South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type(US$ Mn)
Table 11. South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
Table 12. South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
Table 13. Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type(US$ Mn)
Table 14. Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
Table 15. Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
Table 16. Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Software Type(US$ Mn)
Table 17. Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Delivery Mode (US$ Mn)
Table 18. Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By End User (US$ Mn)
Table 19. Organic Developments Done by Companies
Table 20. Recent Inorganic Growth Strategies by the Companies
Table 21. Glossary of Terms, Pharmacovigilance and Drug Safety Software Market
LIST OF FIGURES
Figure 1. Pharmacovigilance and Drug Safety Software Market Segmentation
Figure 2. Asia Pacific Pharmacovigilance and Drug Safety Software Market Overview
Figure 3. Adverse Event Reporting Software Segment Held Largest Share of Software Type in Pharmacovigilance and Drug Safety Software Market
Figure 4. Contract Research Organizations Segment Held Largest Share of End User in Pharmacovigilance and Drug Safety Software Market
Figure 5. China is Expected to Show Remarkable Growth During the Forecast Period
Figure 6. Pharmacovigilance and Drug Safety Software Market - Asia Pacific PEST Analysis
Figure 7. Pharmacovigilance and Drug Safety Software Market Impact Analysis of Driver and Restraints
Figure 8. Asia Pacific Pharmacovigilance and Drug Safety Software Market – Revenue Forecasts and Analysis – 2019- 2027
Figure 9. Pharmacovigilance (PV) and Drug Safety Software Market, by Software Type, 2019 & 2027 (%)
Figure 10. Adverse Event Reporting Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 11. Drug Safety Audits Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 12. Issue Tracking Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 13. Fully Integrated Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 14. Pharmacovigilance (PV) and Drug Safety Software Market, by Delivery Mode 2019 & 2027 (%)
Figure 15. On-premise Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 16. Cloud Based Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 17. Pharmacovigilance (PV) and Drug Safety Software Market, by End User, 2019 & 2027 (%)
Figure 18. Pharmaceutical and Biotech Companies Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 19. Contract Research Organizations (CROs) Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 20. Business Process Outsourcing (BPO) Firms Revenue and Forecasts to 2027 (US$ Mn)
Figure 21. Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue Overview, By Country, 2019 (US$ Mn)
Figure 22. Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027, By Country (%)
Figure 23. Japan Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 24. China Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 25. India Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 26. South Korea Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 27. Australia Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 28. Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Revenue and Forecasts to 2027 (US$ Mn)
Figure 29. Impact of COVID-19 Pandemic in Asia Pacific Country Markets
Figure 30. Growth Strategies in the Pharmacovigilance and Drug Safety Software Market
- Veeva Systems
- IQVIA Inc.
- AB Cube
- ArisGlobal LLC
- Sparta Systems
- Oracle Corporation
- Sarjen Systems Pvt. Ltd
- EXTEDO
- Online Business Applications, Inc.
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players and segments in the Asia Pacific pharmacovigilance and drug safety software market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the Asia Pacific pharmacovigilance and drug safety software market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth Asia Pacific market trends and outlook coupled with the factors driving the pharmacovigilance and drug safety software market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing and distribution